Question 25.10: High- and Low-Spin Octahedral Complexes How many unpaired el...
High- and Low-Spin Octahedral Complexes
How many unpaired electrons are there in the complex ion [Co(NH_3)_5NO_2]^{2+}?
The blue check mark means that this solution has been answered and checked by an expert. This guarantees that the final answer is accurate.
Learn more on how we answer questions.
Learn more on how we answer questions.
| Determining the Number of Unpaired Electrons in Octahedral Complexes | ||
| Begin by determining the charge and number of d electrons on the metal. | SOLUTION
The metal is Co^{3+} and has a d^{6} electronic configuration. |
|
| Look at the spectrochemical series to determine whether the ligand is a strong-field or a weak-field ligand. | NH_3 and NO_2{}^- are both strong-field ligands, so Δ is relatively large. | |
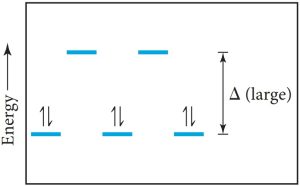
| Decide if the complex is high- or low-spin and draw the electron configuration. | Strong-field ligands yield low-spin configurations.
|
|
| Count the unpaired electrons. | This configuration has no unpaired electrons. | |
Related Answered Questions
Question: 25.9
Verified Answer:
Question: 25.6
Verified Answer:
Question: 25.5
Verified Answer:
Question: 25.2
Verified Answer:
Question: 25.1
Verified Answer:
Question: 25.4
Verified Answer:
Question: 25.3