Question 8.4: Predict the shape and the H–O–H bond angles for a hydronium ......
Predict the shape and the H–O–H bond angles for a hydronium ion, H_{3}O^{+}.
Step-by-Step
The 'Blue Check Mark' means that this solution was answered by an expert.
Learn more on how do we answer questions.
Learn more on how do we answer questions.
The Lewis formula for a H_{3}O^{+} ion is
\begin{bmatrix} H -\overset{..}{O}- H \\\overset{|}{H} \end{bmatrix} ^{\oplus }There are three hydrogen ligands and one lone pair attached to the central oxygen atom. Thus, it belongs to the class AX_{3}E. We see from Table 8.2 that it has a trigonal pyramidal shape. The H–O–H bond angles for a H_{3}O^{+} ion will be somewhat less than that of an ideal tetrahedron and so we predict that the angle is <109.5°.
| TABLE 8.2 Molecular shapes. the white spheres represent the central atom, the red spheres represent ligands, and the green lobes represent lone pairs. | |||||
| Molecular class | Ideal shape | Examples | Molecular class | Ideal shape | Examples |
| AX_{2} |  |
CO_{2},\ HCN,\ BeCl_{2} | AX_{4}E |  |
SF_{4},\ XeO_{2}F_{2},\ IF^{+}_{4}, IO_{2}F^{–}_{2} |
| AX_{3} |  |
SO_{3},\ BF_{3},\ NO_{3}^{–},\ CO_{3}^{2–} | AX_{3}E_{2} | 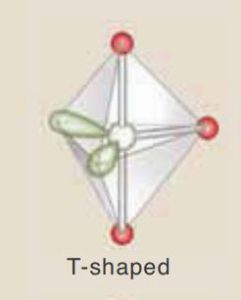 |
CIF_{3},\ BrF_{3} |
| AX_{2}E |  |
SO_{2},\ O_{3},\ PbX_{2},\ SnX_{2} (where X is a halogen) |
AX_{2}E_{3} |  |
XeF_{2},\ I^{–}_{3},\ IF^{–}_{2} |
| AX_{4} |  |
SiH_{4},\ CH_{4},\ SO_{4}^{2–},\ ClO_{4}^{–},\ PO^{3-}_{4},\ XeO_{4} | AX_{6} |  |
SF_{6},\ IOF_{5} |
| AX_{3}E |  |
NH_{3},\ PF_{3},\ AsCl_{3}, ClO^{–}_{3},\ H_{3}O^{+},\ XeO_{3} | AX_{5}E |  |
IF_{5},\ TeF_{5}^{–},\ XeOF_{4} |
| AX_{2}E_{2} |  |
H_{2}O,\ OF_{2},\ SF_{2} | AX_{4}E_{2} |  |
XeF_{4}, ICI_{4}^{-} |
| AX_{5} |  |
PCl_{5},\ AsF_{5},\ SOF_{4} | |||
Related Answered Questions
Question: 8.6
Verified Answer:
The Lewis formulas for these two molecules are
[la...
Question: 8.11
Verified Answer:
(a) As predicted by VSEPR theory, the molecule [la...
Question: 8.10
Verified Answer:
The Lewis formula for a CH_{2}Cl_{2}[/latex...
Question: 8.9
Verified Answer:
The Lewis formula for a XeF_{4}(s) ...
Question: 8.8
Verified Answer:
The Lewis formula for an \mathrm{I}_3^{-}[/...
Question: 8.7
Verified Answer:
There are three equivalent resonance forms of a [l...
Question: 8.5
Verified Answer:
The Lewis formula for a H_{2}CO mol...
Question: 8.3
Verified Answer:
The Lewis formula for a PCl_{6}^{–}...
Question: 8.2
Verified Answer:
The Lewis formula for an NH_{4}^{+}...
Question: 8.1
Verified Answer:
The Lewis formula for a silane molecule is
...