Question 26.SE.3: Draw all the structural and geometric isomers of pentene, C5...
Draw all the structural and geometric isomers of pentene, C_5H_{10}, that have an unbranched hydrocarbon chain.
Learn more on how we answer questions.
Analyse We are given the name of an alkene and asked to derive all possible isomer permutations.
Plan Because the compound is named pentene and not pentadiene or pentatriene, we know that the five-carbon chain contains only one carbon–carbon double bond. Thus we can begin by first placing the double bond in various locations along the chain, remembering that the chain can be numbered from either end. After finding the different distinct locations for the double bond, we can consider whether the molecule can have cis and trans isomers.
Solve There can be a double bond after either the first carbon (pent-1-ene) or second carbon (pent-2-ene). These are the only two possibilities because the chain can be numbered from either end. (Thus what we might erroneously call pent-4-ene is actually pent-1-ene, as seen by numbering the carbon chain from the other end.) Because the first C atom in pent-1-ene is bonded to two H atoms, there are no cis–trans isomers. However, there are cis and trans isomers for pent-2-ene. Thus the three isomers for pentene are
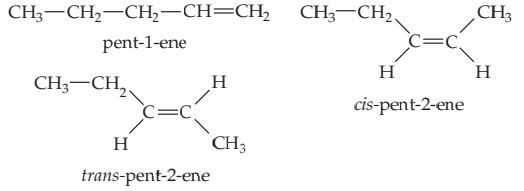
(You should convince yourself that cis- or trans-pent-3-ene is identical to cis- or trans-pent-2-ene, respectively.)