Question 13.9: Write the structure of a segment of polystyrene, used in foa...
Write the structure of a segment of polystyrene, used in foams and molded plastics. The monomer is

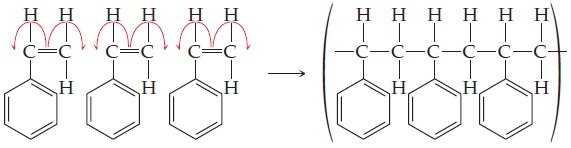
ANALYSIS The polymerization reaction resembles the addition of two monomer units to either end of the double bond.
The blue check mark means that this solution has been answered and checked by an expert. This guarantees that the final answer is accurate.
Learn more on how we answer questions.
Learn more on how we answer questions.
Draw three molecules of styrene with the double bonds aligned next to each other; then add the monomer units together with single bonds, eliminating the double bonds in the process.
Related Answered Questions
Question: 13.11
Verified Answer:
Since all carbons in the benzene ring are equivale...
Question: 13.10
Verified Answer:
The parent compound is a benzene ring with an amin...
Question: 13.8
Verified Answer:
Because this is not an unsymmetrically substituted...
Question: 13.7
Verified Answer:
\underset{\text{3-Methylbut-1-ene}}{\begin{...
Question: 13.6
Verified Answer:
\left(\text{same as}\qquad \begin{matrix} \...
Question: 13.5
Verified Answer:
The reaction is
CH _3 CH _2 CH _2 CH=CHCH _...
Question: 13.4
Verified Answer:
(a) Two H atoms have been added in place of the do...
Question: 13.3
Verified Answer:
Trace the longest chain in each structure. In the ...
Question: 13.2
Verified Answer:
STEP 1: The parent compound is a five-carbon chain...
Question: 13.1
Verified Answer:
STEP 1: The longest continuous chain containing th...