Question 14.7: Glyceraldehyde-3-phosphate is a key intermediate in the meta...
Glyceraldehyde-3-phosphate is a key intermediate in the metabolism of glucose (both glycolysis and gluconeogenesis). Determine which (if any) of the carbons in this molecule are chiral (The carbons have been numbered for clarity).
ANALYSIS Identify the tetrahedral carbons in the molecule; a carbon will be chiral if it is tetrahedral AND is bonded to four different groups.

Learn more on how we answer questions.
We can ignore C1, as it is not tetrahedral (carbons that are part of a double bond are trigonal planar). List the groups attached to each of the remaining carbon atoms.
\begin{array}{ll}\text{Groups on Carbon 2}& \text{Groups on Carbon}3 \\\hline \text{1.}- CHO & \text{1.}- CH ( OH ) CHO \\\text{2.}- OH & \text{2.}- H \\\text{3.}- H & \text{3.}- H \\\text{4.}- CH _2 OPO _3 H _2 & \text{4.}- CH _2 OPO _3 H _2 \\\hline\end{array}
Looking at the lists we see that only carbon 2 has four different groups attached. Therefore, only C2 is chiral.
(b) 2-Deoxyribose is a carbohydrate that makes up the backbone of the biomolecule ribonucleic acid (RNA). Determine which (if any) of the carbons in this molecule are chiral (The carbons have been numbered for clarity).
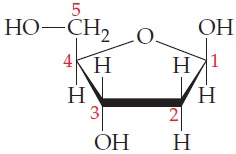
ANALYSIS As in part (a), begin by identifying the tetrahedral carbons in the molecule and then list what is attached; use R, R′, and R″ to represent different carbon chains when the groups are complex and not as easy to list.
When dealing with molecules in line structure format, it is sometimes easier to actually put the carbons in to avoid confusion:

All carbons in this molecule are tetrahedral. List the groups attached to each of the remaining carbon atoms; indicate different complex carbon chains beyond the carbon adjacent to that being examined by using R and R′. Analyze each of the carbons one at a time to determine chirality:
\begin{array}{lllll}\text{Groups on C1}& \text{Groups on C2}& \text{Groups on C3}& \text{Groups on C4}& \text{Groups on C5}\\\hline \text{1.}- OR & \text{1.}- CH ( OH ) R & \text{1.}- CH _2 R ^{\prime}& \text{1.}- CH ( OH ) R & \text{1.}- OH \\\text{2.}- OH & \text{2.}- H & \text{2.}- OH & \text{2.}- CH _2 OH & \text{2.}- H \\\text{3.}- H & \text{3.}- H & \text{3.}- H & \text{3.}- H & \text{3.}- H \\\text{4.}- CH _2 R ^{\prime}& \text{4.}- CH ( OH ) R ^{\prime}& \text{4.}- CH \left( CH _2 OH \right) OR & \text{4.}- OR ^{\prime}& \text{4.}- CH ( R )\left( R ^{\prime}\right) \\\hline\end{array}
Comparing the groups on each carbon, we see that C2 and C5 are both achiral; note that both have only three different groups attached (also note that on C2 the —CH_(OH)R and —CH(OH)R′ are different, noted by the use of R and R′). The other carbons have four different groups attached. Therefore, C1, C3, and C4 are chiral.