Question 27.1: A solution consisting of 30 percent MgSO4 and 70 percent H2O......
A solution consisting of 30 percent MgSO4 and 70 percent H2O is cooled to 60°F. During cooling, 5 percent of the total water in the system evaporates. How many kilograms of crystals are obtained per kilogram of original mixture?
Learn more on how we answer questions.
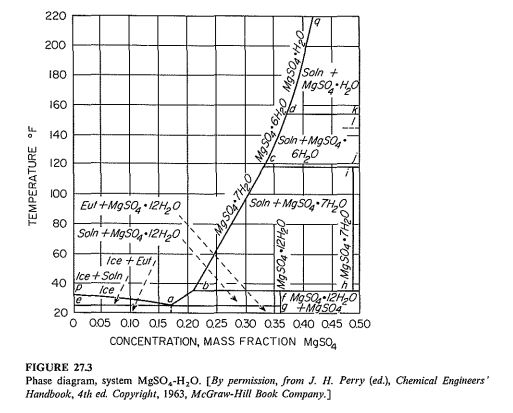
From Fig. 27.3 it is noted that the crystals are MgSO4⋅7H2O and that the concentration of the mother liquor is 24.5 percent anhydrous MgSO4 and 75.5 percent H2O. Per 1000 kg of original solution, the total water is 0.70 × 1000 = 700 kg. The evaporation is 0.05 × 700 = 35 kg. The molecular weights of MgSO4 and MgSO4⋅7H2O are 120.4 and 246.5, respectively, so the total MgSO4⋅7H2O in the batch is 1000 × 0.30(246.5/120.4) = 614 kg, and the free water is 1000- 35 – 614 = 351 kg. In 100 kg of mother liquor, there is 24.5(246.5/120.4) = 50.16 kg of MgSO4⋅7H2O and 100- 50.16 = 49.84 kg of free water. The MgSO4⋅7H2O in the mother liquor, then, is (50.16/49.84)351 = 353 kg. The final crop is 614-353 = 261 kg.