A cylinder having an initial volume of 100 ft ^{3} contains 0.2 lbm of water at 100 F. The water is then compressed in an isothermal quasi-equilibrium process until it has a quality of 50%. Calculate the work done in the process assuming water vapor is an ideal gas.
Question 3.300E: A cylinder having an initial volume of 100 ft^3 contains 0.2...
The Blue Check Mark means that this solution has been answered and checked by an expert. This guarantees that the final answer is accurate.
Learn more on how we answer questions.
Learn more on how we answer questions.
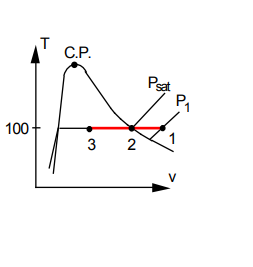
State 1: T _{1}, \quad v _{1}= V / m =\frac{100}{0.2}=500 ft ^{3} / lbm \quad\left(> v _{ g }\right)
since P _{ g }=0.95 psia, very low so water is an ideal gas from 1 to 2.
P _{1}= P _{ g } \times \frac{ v _{ g }}{ v _{1}}=0.950 \times \frac{350}{500}=0.6652 lbf / in ^{2}
V _{2}= mv _{2}=0.2 lbm*350 ft ^{3} / lbm =70 ft ^{3}
v _{3}=0.01613+0.5 \times(350-0.01613)=175.0 ft ^{3} / lbm
For ideal gas and constant T the work term follows Eq. 3.21
{ }_{1} W _{2}=\int PdV = P _{1} V _{1} \ln \frac{ V _{2}}{ V _{1}}=0.6652 \times \frac{144}{778} \times 100 \ln \frac{70}{100}=-4.33 Btu
For the constant pressure part of the process the work becomes
{ }_{2} W _{3}= P _{2} m \left( v _{3}- v _{2}\right)=0.95 psi \times 0.2 lbm \times(175-350) ft ^{3} / lbm \times 144 in ^{2} / ft ^{2}
= −4788 lbf-ft = −6.15 Btu
{ }_{1} W _{3}=-6.15-4.33=- 1 0 . 4 8 ~ B t u
………………………
Eq.3.21 : { }_{1} w _{2}=\frac{P_{2} V_{2}-P_{1} V_{1}}{1-n}

Related Answered Questions
C.V. A + B . Then { }_{1} Q _{2}=\emptys...
C.V. The 1 lbm water.
Continuty: m _{2}=...
C.V. The mass of R-134a. Properties in Table F.10....
C.V. Water. This is a control mass.
m _{2}=...
\dot{ Q }= k A \frac{\Delta T }{\Delta x }[...
Steady state conduction through a single layer boa...
One dimensional heat transfer by conduction, we do...
\dot{ W }= F V =300 lbf \times 40( mi / h...