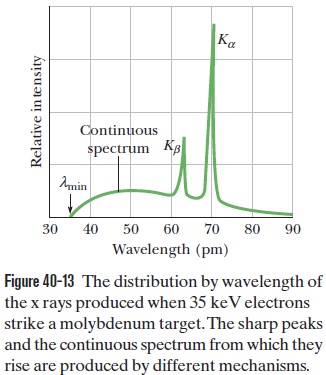
Question 40.42: From Fig. 40-13, calculate approximately the energy differen......
From Fig. 40-13, calculate approximately the energy difference E_L – E_M for molybdenum. Compare it with the value that may be obtained from Fig. 40-15.

Step-by-Step
The 'Blue Check Mark' means that this solution was answered by an expert.
Learn more on how do we answer questions.
Learn more on how do we answer questions.
Using hc = 1240 eV·nm = 1240 keV·pm, the energy difference E_L – E_M for the x-ray atomic energy levels of molybdenum is
\Delta E=E_L-E_M=\frac{h c}{\lambda_L}-\frac{h c}{\lambda_M}=\frac{1240 \,keV \cdot pm }{63.0 \, pm }-\frac{1240 \,keV \cdot pm }{71.0 \,pm }=2.2 \,keV .
Related Answered Questions
Question: 40.38
Verified Answer:
From the data given in the problem, we calculate f...
Question: 40.37
Verified Answer:
Suppose an electron with total energy E and moment...
Question: 40.39
Verified Answer:
THINK The frequency of an x-ray emission is propor...
Question: 40.40
Verified Answer:
(a) According to Eq. 40-26, f \propto(Z-1)...
Question: 40.41
Verified Answer:
We use Eq. 36-31, Eq. 39-6, and hc = 1240 eV·nm = ...
Question: 40.43
Verified Answer:
(a) An electron must be removed from the K-shell, ...
Question: 40.44
Verified Answer:
(a) and (b) Let the wavelength of the two photons ...
Question: 40.45
Verified Answer:
The initial kinetic energy of the electron is K[la...
Question: 40.46
Verified Answer:
The transition is from n = 2 to n = 1, so Eq. 40-2...
Question: 40.47
Verified Answer:
Let the power of the laser beam be P and the energ...