How many grams of aspirin are contained in 50.0 mL of a 0.050 M solution?
Analysis
Use the molarity to convert the volume of the solution to moles of solute. Then use the molar mass to convert moles to grams.
Question 7.sp.9: How many grams of aspirin are contained in 50.0 mL of a 0.05...
The Blue Check Mark means that this solution has been answered and checked by an expert. This guarantees that the final answer is accurate.
Learn more on how we answer questions.
Learn more on how we answer questions.
[1] Identify the known quantities and the desired quantity.
0.050 M ? g aspirin
50.0 mL solution
known quantities desired quantity
[2] Determine the number of moles of aspirin using the molarity.
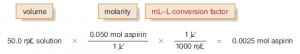
[3] Convert the number of moles of aspirin to grams using the molar mass (180.2 g/mol).

Related Answered Questions
Identify the known quantities and the desired quan...
a. The greater the number of dissolved particles, ...
[1] Identify the known quantities and the desired ...
[1] Identify the known quantities and the desired ...
[1] Identify the known quantities and the desired ...
[1] Convert milligrams of DDT to grams of DDT so t...
[1] Identify the known quantities and the desired ...
[1] Identify the known quantities and the desired ...
(v/v)% =\frac {101 mL ethanol}{750 mL win...