Question 13.10: Formal Charges Give possible Lewis structures for XeO3, an e...
Formal Charges
Give possible Lewis structures for XeO _{3} , an explosive compound of xenon. Determine the formal charges of each atom in the various Lewis structures.
The blue check mark means that this solution has been answered and checked by an expert. This guarantees that the final answer is accurate.
Learn more on how we answer questions.
Learn more on how we answer questions.
For XeO _{3} (26 valence electrons) we can draw the following possible Lewis structures (formal charges are indicated in parentheses):
 |
 |
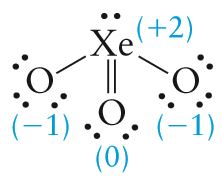 |
 |
 |
 |
 |
 |
Related Answered Questions
Question: 13.13
Verified Answer:
The traditional Lewis structure for Xe F_{4...
Question: 13.14
Verified Answer:
First, we must determine the Lewis structure for t...
Question: 13.12
Verified Answer:
In the traditional Lewis structure for PC l...
Question: 13.2
Verified Answer:
The HCl molecule: Because the electronegativity of...
Question: 13.11
Verified Answer:
The Lewis structure for water is
There are four p...
Question: 13.9
Verified Answer:
a. The chlorine atom (third row) accepts the extra...
Question: 13.8
Verified Answer:
We can follow the same stepwise procedure we used ...
Question: 13.7
Verified Answer:
We will follow the usual procedure for obtaining t...
Question: 13.5
Verified Answer:
The idea here is to break the bonds in the reactan...
Question: 13.6
Verified Answer:
In each case we apply the three rules for writing ...