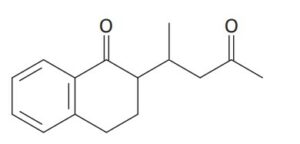
Question 17.9: How might you use an enamine reaction to prepare the followi...
How might you use an enamine reaction to prepare the following compound?
S t r a t e g y
The overall result of an enamine reaction is the Michael addition of a ketone as donor to an α,β-unsaturated carbonyl compound as acceptor, yielding a 1,5-dicarbonyl product. The C–C bond made in the Michael addition step is the one between the α carbon of the ketone donor and the β carbon of the unsaturated acceptor.
The blue check mark means that this solution has been answered and checked by an expert. This guarantees that the final answer is accurate.
Learn more on how we answer questions.
Learn more on how we answer questions.

Related Answered Questions
Question: 17.1
Verified Answer:
The acidity order is (a)\gt (c)\gt (b)[/lat...
Question: 17.8
Verified Answer:
Question: 17.4
Verified Answer:
Question: 17.2