Question 9.9: Draw the Lewis structure (including resonance structures) fo...
Draw the Lewis structure (including resonance structures) for nitromethane (CH_{3}NO_{2}). For each resonance structure, assign formal charges to all atoms that have formal charge.
The blue check mark means that this solution has been answered and checked by an expert. This guarantees that the final answer is accurate.
Learn more on how we answer questions.
Learn more on how we answer questions.
| Begin by writing the skeletal structure. For organic compounds, the condensed structural formula (in this case CH_{3}NO_{2}) indicates how the atoms are connected. |  |
| Calculate the total number of electrons for the Lewis structure by summing the number of valence electrons for each atom. | Total number of electrons for Lewis structure = # valence e^{-} in C + 3(# valence e^{-} in H) + # valence e^{-} in N +2(# valence e^{-} in O) = 4 + 3(1) + 5 + 2(6) = 24 |
| Place a dash between each pair of atoms to indicate a bond. Each dash counts for two electrons. | 
(12 of 24 electrons used) |
| Distribute the remaining electrons, first to terminal atoms, then to interior atoms. | 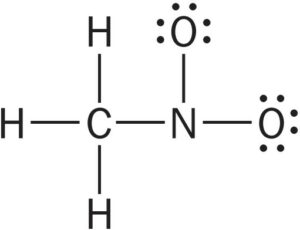 (24 of 24 electrons used) |
| If there are not enough electrons to complete the octets on the interior atoms, form double bonds by moving lone pair electrons from terminal atoms into the bonding region with interior atoms. |  |
| Draw any necessary resonance structures by moving only electron dots. (In this case, you can form a double bond between the nitrogen atom and the other oxygen atom.) |  |
| Assign formal charges (FC) to each atom. FC = # valence e^{-} -(# nonbonding e^{-}+\frac{1}{2} # bonding e^{-}) |

Carbon, hydrogen, and the doubly bonded oxygen atoms have no formal charge. Nitrogen has a +1 formal charge [5 – \frac{1}{2} (8)], and the singly bonded oxygen atom in each resonance structure has a -1 formal charge [6-(6 +\frac{1}{2} (2)]. |
Related Answered Questions
Question: 9.8
Verified Answer:
Calculate the formal charge on each atom by findin...
Question: 9.6
Verified Answer:
Begin by writing the skeletal structure.
Since hyd...
Question: 9.5
Verified Answer:
Since hydrogen is always terminal, put nitrogen in...
Question: 9.4
Verified Answer:
Because carbon is the less electronegative atom, p...
Question: 9.11
Verified Answer:
Begin by rewriting the reaction using the Lewis st...
Question: 9.10
Verified Answer:
Begin by writing the skeletal structure. Since flu...
Question: 9.7
Verified Answer:
Begin by writing the skeletal structure. Since nit...
Question: 9.3
Verified Answer:
(a) From Figure 9.7, find the electronegativities ...
Question: 9.2
Verified Answer:
KBr and KCl have lattice energies of smaller magni...
Question: 9.1
Verified Answer:
Draw Lewis symbols for calcium and chlorine based ...