Question 10.2: Predicting Molecular Geometries Predict the geometry and bon...
Predicting Molecular Geometries
Predict the geometry and bond angles of PCl_{3}.
The blue check mark means that this solution has been answered and checked by an expert. This guarantees that the final answer is accurate.
Learn more on how we answer questions.
Learn more on how we answer questions.
PCl_{3} has 26 valence electrons.
:\overset{..}{\underset{..}{Cl}}-\overset{:\overset{..}{\underset{\mid }{Cl}} :}{\underset{..}{P}}- \overset{..}{\underset{..}{Cl}:}The central atom (P) has four electron groups.
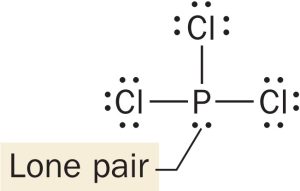
Three of the four electron groups around P are bonding groups and one is a lone pair.
The electron geometry is tetrahedral (four electron groups), and the molecular geometry—the shape of the molecule—is trigonal pyramidal (three bonding groups and one lone pair). Because of the presence of a lone pair, the bond angles are less than 109.5°.

Related Answered Questions
Question: 10.3
Verified Answer:
ICl_{4}^{-} has 36 valence electron...
Question: 10.6
Verified Answer:
BrF_{3} has 28 valence electrons an...
Question: 10.7
Verified Answer:
Acetaldehyde has 18 valence electrons and the foll...
Question: 10.4
Verified Answer:
Begin by drawing the Lewis structure of CH_...
Question: 10.10
Verified Answer:
Write an energy-level diagram for the molecular or...
Question: 10.9
Verified Answer:
The H_{2}^{-} ion has three electro...
Question: 10.8
Verified Answer:
1. Write the Lewis structure for the molecule.
...
Question: 10.5
Verified Answer:
Draw the Lewis structure for the molecule and dete...
Question: 10.1
Verified Answer:
Determine the molecular geometry of NO_{3}^...