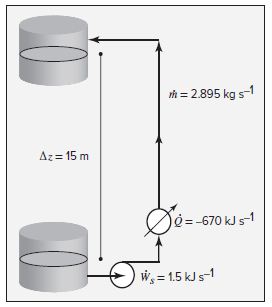
Question 2.14: Water at 90°C is pumped from a storage tank at a rate of 3 L...
Water at 90°C is pumped from a storage tank at a rate of 3 L·s^{−1}. The motor for the pump supplies work at a rate of 1.5 kJ·s^{−1}. The water goes through a heat exchanger, giving up heat at a rate of 670 kJ·s^{−1} , and is delivered to a second storage tank at an elevation 15 m above the first tank. What is the temperature of the water delivered to the second tank?
Learn more on how we answer questions.
This is a steady-state, steady-flow process for which Eq. (2.31) applies. The initial and final velocities of water in the storage tanks are negligible, and the term Δu^{2}/2
can be omitted. All remaining terms are expressed in units of kJ·kg^{−1} . At 90°C the density of water is 0.965 kg·L^{−1} and the mass flow rate is:
\Delta H + \frac{\Delta u^2}{2} + g \Delta z=Q+W_s (2.31)
\dot{m}=(3)(0.965)= 2.895 kg⋅s^{-1}
For the heat exchanger,
Q = − 670 / 2.895 = − 231.4 kJ⋅ kg^{−1}
For the shaft work of the pump,
W_{s}=1.5 / 2.895 = 0.52 kJ⋅ kg ^{−1}
If g is taken as the standard value of 9.8 m·s^{−2} , the potential-energy term is:
g\Delta z= (9.8) (15)= 147 m^{2} .s^{-2}= 147 J⋅ kg^{−1} = 0.147 kJ⋅ kg^{−1}
The steam-table value for the enthalpy of liquid water at 90°C is:
H_{1} = 376.9 kJ⋅ kg ^{−1}
Thus,
ΔH = H_{2} − H_{1} = H_{2} − 376.9 = − 231.0
and
H_{2} = 376.9 − 231.0 = 145.9 kJ⋅ kg ^{−1}
The temperature of water having this enthalpy is found from the steam tables:
t = 34.83° C
In this example, W_{s} and gΔz are small compared with Q, and for practical purposes could have been neglected.