Question 4.11: Draw the structural formula of the major organic product(s)......
Draw the structural formula of the major organic product(s) formed in the following reactions. Indicate the stereochemistry where appropriate.





Learn more on how we answer questions.
(a) Hydrogen bromide adds to the double of alkene to yield alkyl bromide.

(c) Attack of an acid on alkene leads to the formation of tertiary carbocation which on 1, 2-alkyl shift followed by hydrolysis gives alcohol.
![]()
(d) Cyclopentane in the presence of halogen and UV light form alkyl halide by free radical mechanism. This on dehydrohalo- genation in presence of base generates alkene. When this alkene reacts with bromine in bromoform, anti-addition occurs, and the product is trans-1, 2-dibromocyclopentane.
![]()

(g) Protonation of alkene forms a carbocation intermediate which upon rearrangement and halide attack gives alkyl halide.
![]()
(h) Attack of proton on alkene generates a tertiary carbocation that on the attack of internal nucleophile forms five membered intermediate that yields the product on the loss of proton.

(j) Attack of halide on carbocation can occur by anti- and syn- addition.

(k) Cycloalkene on hydrogenation over palladium forms cycloalkane. It undergoes chlorination by free radical mechanism in the presence of UV light to form cycloalkyl halide.
![]()
![]()

(m) If the halogenation of an alkene is carried out in aqueous solution, the major product of the overall reaction is not a vicinal- dihalide; instead, a halohydrin is formed. In this case, molecules of the solvent also act as reactants.

(n) This is an example of hydroboration-oxidation reaction on an alkene. The first step is the addition of a boron atom and a hydrogen atom to the double bond, called hydroboration; the second step is oxidation and hydrolysis of the alkylborane intermediate to form an alcohol and boric acid.
![]()
(o) In the first step, polarization of bromine by alkene occurs that leads to a bromonium ion intermediate. In the next step, an iodide anion attacks at the back side of one carbon of the bromonium ion in an S_N2 reaction, causing the ring to open and resulting in the formation of vicinal dihalide.

(p) Alkenes react with mercuric acetate in a mixture of tetrahydrofuran (THF) and phenol to produce (alkylphenoxy) mercury compounds, that can further be reduced with sodium borohydride.
![]()
(q) This is an example Simmons-Smith reaction.
![]()
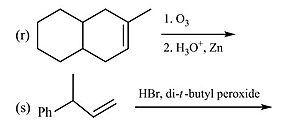
(r) The first step involves ozonolysis of alkene followed by workup with zinc and acid which results in oxidative cleavage.

(s) In the presence of peroxide, hydrogen bromide undergoes anti-Markovnikov addition to carbon-carbon double bond.
![]()
(t) Alkenes react with diazomethane to give cyclopropane. A carbene such as methlyene is generated that attacks the alkene to break the double bond and form cyclopropane.

(u) The given alkyl halide in the presence of base undergoes elimination to form alkenes. Treatment of alkene with osmium tetroxide involves the formation of cyclic intermediate that result in syn-addition of the oxygen atoms. The cyclic inter- mediate undergoes cleavage at the oxygen-metal bonds, without altering the stereochemistry of the two new C-O bond.

(v) Iodine azide adds to an alkene through a three-membered cyclic iodonium ion intermediate.

(w) Protonation of an alkene leads to resonance stabilized intermediate which on further attack by nucleophile yields the product.

(x) Protonation followed by rearrangement of double bonds of alkene gives the product obtained.

(y) Dehydration of alcohol in the presence of strong acid like sulphuric acid forms alkene. It undergoes oxidation with potassium permanganate to form 1,2-diol.

(z) Protonation of the epoxide causes the opening of ring due to attack of double bond on electrophilic carbon. Subsequent rearrangement and loss of proton leads to the desired product.
