Question 15.9: Balancing a Net Ionic Redox Equation Using the Oxidation Num......
Balancing a Net Ionic Redox Equation Using the Oxidation Number Method
Balance the following net ionic redox equation for the reaction between MnO {_4}^- and C _2 O {_4}^{2-} in basic solution using the oxidation number method for balancing redox equations.
MnO {_4}^- + C _2 O {_4}^{2-} \longrightarrow MnO _2 + CO {_3}^{2-}
Learn more on how do we answer questions.
Step 1 The elements being oxidized and reduced are identified by assigning oxidation numbers.
MnO {_4}^- + C _2 O {_4}^{2-} \longrightarrow MnO _2 + CO {_3}^{2-}
+7 +2 +3 -2 +4 -2 +4 -2
The two elements undergoing oxidation number change are Mn and C.
Step 2 The change in oxidation number per atom is determined.

Step 3 The carbon oxidation number change is multiplied by 2, since there are two carbon atoms in the C _2 O {_4}^{2-} , to obtain the oxidation number change per formula unit.
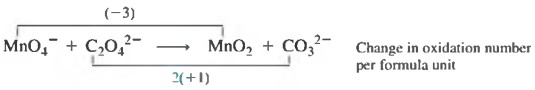
Step 4 By multiplying the Mn per formula unit oxidation number decrease of -3 by 2 and multiplying the C per formula unit oxidation number increase of +2 by 3, the oxidation number increase and the oxidation number decrease become equal; both are at six units.

Step 5 The bracket notation indicates that two Mn atoms and six C atoms undergo an oxidation number change. Translating this information into coefficients produces the equation
4 MnO {_4}^- + 3 C _2 O {_4}^{2-} \longrightarrow 2 MnO _2 + 6 CO {_3}^{2-}
Step 6 The only atoms left to balance are those of O.
Step 7 Since this is a net ionic equation, the ionic charges must balance. In a basic solution, which is the situation in this example, charge balance is accomplished by adding OH ^- ions.
The equation, at present, has a net charge on the reactant side of -8 (two -1 ions and three -2 ions). The charge on the product side of the equation is -12 (six -2 ions). By adding four OH ^- ions to the reactant side, the charge balances at -12 on each side of the equation.
The equation, with charge balance included, becomes
\frac{2 {\textrm{ MnO}_4}^- + 3 \textrm{ C}_2 {\textrm{O}_4}^{2-} + \textbf{4 OH}^-}{-12 \textrm{ charge}} \longrightarrow \frac{2 \textrm{ MnO}_2 + 6 {\textrm{ CO}_3}^{2-}}{-12 \textrm{ charge}}Step 8 H atom balance is achieved by addition of H _2 O molecules. Two H _2 O molecules are added to the product side of the equation to counterbalance the four H atoms already present on the reactant side of the equation (four OH ^- ions).
4 MnO {_4}^- + 3 C _2 O {_4}^{2-} \longrightarrow 2 MnO _2 + 6 CO {_3}^{2-} + 2 H _2 O
Step 9 The oxygen atoms should automatically balance if all of the previous steps have been carried out correctly. Such is the case. There are 24 O atoms on each side of the equation.
The final balanced equation is
4 MnO {_4}^- + 3 C _2 O {_4}^{2-} \longrightarrow 2 MnO _2 + 6 CO {_3}^{2-} + 2 H _2 O
Answer Double Check: Is the equation balanced relative to both atoms and charge? Yes.
(2 Mn, 6 C, 4 H, 24 O, -12 charge) = (2 Mn, 6 C, 4 H, 24 O, -12 charge)