Question 19.2: Exploring Differences between Second- and Third-Row Elements......
Exploring Differences between Second- and Third-Row Elements
Account for the following observations:
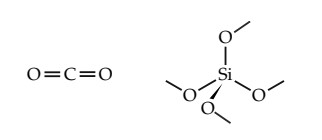
(a) CO_2 is a gaseous molecular substance, whereas SiO_2 is a covalent network solid in which SiO_4 tetrahedra are linked to four neighboring SiO_4 tetrahedra by shared oxygen atoms. (the first photo below)
(b) Glass made of SiO_2 is attacked by hydrofluoric acid with the formation of SiF_6^{2-} anions. The analogous CF_6^{2-} anion does not exist.
STRATEGY
To account for these differences, remember that
(a) second-row atoms are smaller and form stronger multiple bonds than third-row atoms and
(b) third-row atoms can form more than four bonds because of their larger size.

Learn more on how do we answer questions.
(a) Because of its small size and good π overlap with other small atoms, carbon forms strong double bonds with two oxygens to give discrete CO_2 molecules. Because the larger Si atom does not have good π overlap with other atoms, it uses its four valence electrons to form four single bonds rather than two double bonds.
(b) The larger silicon atom can bond to six F^- ions, whereas the smaller carbon atom can form a maximum of only four bonds.