Question 27.2: Identifying Electrophiles, Nucleophiles, Leaving Groups, Aci......
Identifying Electrophiles, Nucleophiles, Leaving Groups, Acids, and Bases
It is important to be able to distinguish when an electron pair donor in a reaction is acting as a base or as a nucleophile. The following reactions are elementary reactions. In each case, determine whether the electron pair donor is acting as a Brønsted–Lowry base or as a nucleophile. Then, identify the acid, electrophile, and leaving group, as appropriate. Finally, use arrows to indicate the movement of electrons.
(a) CH_{3}S^{−} + CH_{3}CH_{2}O—\overset{\begin{matrix} O \\ \parallel \end{matrix}}{\underset{\begin{matrix} \parallel \\ O \end{matrix} }{S}}—CH_{3}\longrightarrow CH_{3}SCH_{2}CH_{3} + ^{- }O—\overset{\begin{matrix} O \\ \parallel \end{matrix}}{\underset{\begin{matrix} \parallel \\ O \end{matrix} }{S}}—CH_{3}
(b) 
(c) 
Learn more on how do we answer questions.
Analyze
First, we put lone pairs on the atoms, and then compare reactants and products to determine which bonds are formed and which are broken. We identify the electron pair donor and the electron pair acceptor. If the electron pair donor (the attacking species) forms a new bond with a proton, it is acting as Brønsted–Lowry base. If the attacking species forms a new bond with a carbon atom, then it is acting a nucleophile.
Solve
(a) In the equation below, reactants and products are shown with all lone pairs. We see that a new bond is formed between sulfur and carbon, and a bond between carbon and oxygen is broken. CH_{3}S^{−} is acting as a nucleophile, CH_{3}CH_{2}OSO_{2}CH_{3} is the electrophile (or substrate), and ^{- }OSO_{2}CH_{3} is the leaving group. The red arrows indicate the movement of electrons.

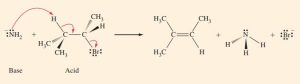
(b) In this reaction, NH_{2}^{- } reacts to form NH_{3}. Because the chemical formulas of these species differ by a single proton, we know that NH_{3} and NH_{2}^{- } are a conjugate acid–base pair. NH_{2}^{- } is acting a Brønsted–Lowry base and (CH_{3})_{2}CHCHBrCH_{3} is acting as an acid.
(c) We see from the equation below that a new carbon–oxygen bond is formed and a carbon–chlorine bond is broken. The HCOO^{−} ion is acting as a nucleophile, CH_{3}CH_{2}Cl is the electrophile, and Cl^{−} is the leaving group.

Assess
We can assess whether or not the substitution reactions in this example are feasible. For example, in (a), we can say that ^{- }OSO_{2}CH_{3} is a weaker (more stable) base than CH_{3}S^{−} because the negative charge in ^{- }OSO_{2}CH_{3} is highly delocalized; it is shared equally by three electronegative oxygen atoms. Thus, equilibrium favors products and we predict that the substitution will occur to a significant extent. For (c), we must compare the stabilities of the bases HCOO^{−} and Cl^{−}. The conjugate acid of HCOO^{−} is HCOOH, a weak acid, and so HCOO^{−} is a weak base. Cl^{−} is an extremely weak base (pK_{b} ≈ 21). Because the weaker base appears on the right side of the equation, we expect reaction (c) will occur as written.