Question 12.8: Are the following pairs of compounds the same (conformers), ...
Are the following pairs of compounds the same (conformers), isomers, or unrelated?
(a) \begin{array}{r c}\underset{|}{\quad \; CH_3} \\ CH_3CHCH_2CH_2 \\ \overset{|}{\quad \; CH_3} \quad \quad \quad \enspace \> \end{array} \begin{array}{r c} \underset{|}{\quad \; CH_3} \quad \quad \quad \quad \quad \enspace \> \\ CH_3CHCH_2CH_2CH_3 \\ \end{array}
(b) \begin{array}{r c} \\ CH_3CH_2CHCH_3 \enspace \, \\ \quad \overset{|}{\quad \quad \quad \; CH_2CH_3} \> \end{array} \begin{array}{r c} \quad \quad \quad \enspace \underset{|}{\quad \qquad \enspace CH_2CH_3} \\ CH_3CHCH_2 \quad \quad \\ \overset{|}{\quad \; CH_3} \qquad \quad \enspace \> \end{array}
(c) \begin{array}{r c} \\ CH_3CH_2OCH_3 \> \end{array} \begin{array}{r c} \underset{||}{O} \quad \\ CH_3CH_2CH \> \end{array}
ANALYSIS First compare molecular formulas to see if the compounds are related, and then look at the structures to see if they are the same compound or isomers. Find the longest continuous carbon chain in each, and then compare the locations of the substituents connected to the longest chain.
Learn more on how we answer questions.
(a) Both compounds have the same molecular formula (C_6H_{14}) so they are related. Since the – CH_3 group is on the second carbon from the end of a 5-carbon chain in both cases, these structures represent the same compound and are conformers of each other.
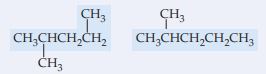
(b) Both compounds have the same molecular formula (C_6H_{14}) and the longest chain in each is 5 carbon atoms. A comparison shows, however, that the – CH_3 group is on the middle carbon atom in one structure and on the second carbon atom in the other. These compounds are isomers of each other.

(c) These compounds have different formulas (C_3H_{8}O and C_3H_{6}O), so they are unrelated; they are neither conformers nor isomers of each other.