Question 13.2: Bond Polarity and Dipole Moment For each of the following mo...
Bond Polarity and Dipole Moment
For each of the following molecules, show the direction of the bond polarities. Also indicate which ones have dipole moments: HCl, Cl_{2}, SO_{3} (planar), CH_{4} (tetrahedral), and H_{2}S (V-shaped).
Learn more on how we answer questions.
The HCl molecule: Because the electronegativity of chlorine (3.2) is greater than that of hydrogen (2.2), the chlorine is partially negative, and the hydrogen is partially positive. The HCl molecule has a dipole moment oriented as follows:

The Cl_{2} molecule: Because the two chlorine atoms share the electrons equally, no bond polarity occurs. The Cl_{2} molecule has no dipole moment.
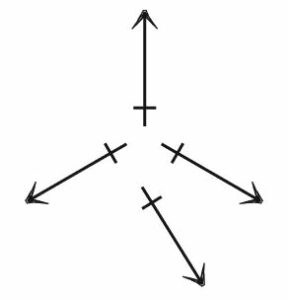
The SO_{3} molecule: Because the electronegativity of oxygen (3.4) is greater than that of sulfur (2.6), each oxygen has a partial negative charge, and the sulfur has a partial positive charge:

However, the bond polarities cancel, and the molecule has no dipole moment.
The C H_{4} molecule: Carbon has a slightly higher electronegativity (2.6) than hydrogen (2.2). This leads to small partial positive charges on the hydrogen atoms and a small partial negative charge on the carbon:

This case is similar to the third type in Table 13.4. Since the bond polarities cancel, the molecule has no dipole moment.
The H_{2}S molecule: Since the electronegativity of sulfur (2.6) is greater than that of hydrogen (2.2), the sulfur has a partial negative charge, and the hydrogen atoms have a partial positive charge, which can be represented as follows:

This case is analogous to the water molecule. The polar bonds result in a dipole moment oriented as shown.

Table 13.4
Types of Molecules with Polar Bonds but no Resulting Dipole Moment
|
Type |
Cancellation of Polar Bonds |
Example |
Ball-and-Stick Model |
||
| Linear molecules with two identical bonds | B—A—B |  |
CO _{2} |  |
|
| Planar molecules with three identical bonds 120 degrees apart |  |
 |
SO _{3} |  |
|
| Tetrahedral molecules with four identical bonds 109.5 degrees apart |  |
 |
CCl _{4} |  |
|