Question 9.3: Classify the bond formed between each pair of atoms as coval...
Classify the bond formed between each pair of atoms as covalent, polar covalent, or ionic.
(a) Sr and F (b) N and Cl (c) N and O
The blue check mark means that this solution has been answered and checked by an expert. This guarantees that the final answer is accurate.
Learn more on how we answer questions.
Learn more on how we answer questions.
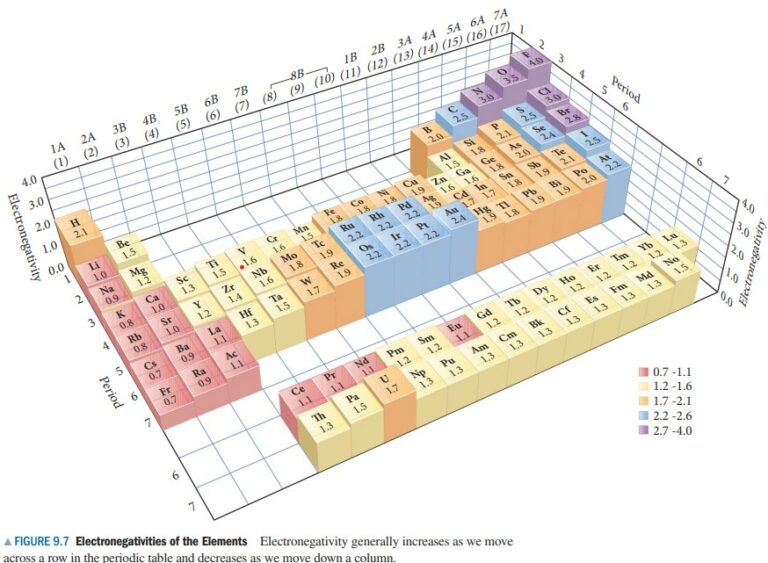
(a) From Figure 9.7, find the electronegativities of Sr (1.0) and of F (4.0). The electronegativity difference is ∆EN = 4.0 – 1.0 = 3.0. Using Table 9.1, classify this bond as ionic.
(b) From Figure 9.7, find the electronegativities of N (3.0) and of Cl (3.0). The electronegativity difference (∆EN) is ∆EN = 3.0 – 3.0 = 0. Using Table 9.1, classify this bond as covalent.
(c) From Figure 9.7, find the electronegativities of N (3.0) and of O (3.5). The electronegativity difference (∆EN) is ∆EN = 3.5 – 3.0 = 0.5. Using Table 9.1, classify this bond as polar covalent.
| TABLE 9.1 The effect of electronegativity Difference on Bond Type | ||
| Electronegativity Difference (∆EN) | Bond Type | Example |
| Small (0-0.4) | Covalent | Cl_{2} |
| Intermediate (0.4-2.0) | Polar covalent | HCl |
| Large (2.0+) | Ionic | NaC |
Related Answered Questions
Question: 9.8
Verified Answer:
Calculate the formal charge on each atom by findin...
Question: 9.6
Verified Answer:
Begin by writing the skeletal structure.
Since hyd...
Question: 9.5
Verified Answer:
Since hydrogen is always terminal, put nitrogen in...
Question: 9.4
Verified Answer:
Because carbon is the less electronegative atom, p...
Question: 9.11
Verified Answer:
Begin by rewriting the reaction using the Lewis st...
Question: 9.10
Verified Answer:
Begin by writing the skeletal structure. Since flu...
Question: 9.9
Verified Answer:
Begin by writing the skeletal structure...
Question: 9.7
Verified Answer:
Begin by writing the skeletal structure. Since nit...
Question: 9.2
Verified Answer:
KBr and KCl have lattice energies of smaller magni...
Question: 9.1
Verified Answer:
Draw Lewis symbols for calcium and chlorine based ...