Question 11.5: Determine whether each of the following molecules can exist ...
Determine whether each of the following molecules can exist as cis–trans isomers: (a) 1-pentene, (b) 3-ethyl-3-hexene, and (c) 3-methyl-2-pentene.
Learn more on how we answer questions.
a. Examine the structure of 1-pentene,

We see that carbon-1 is bonded to two hydrogen atoms, rather than to two different substituents. In this case there can be no cis-trans isomers.
b. Examine the structure of 3-ethyl-3-hexene:

We see that one of the carbons of the carbon-carbon double bond is bonded to two ethyl groups. As in example (a), because this carbon is bonded to two identical groups, there can be no cis or trans isomers of this compound.
c. Finally, examination of the structure of 3-methyl-2-pentene reveals that both a cis and trans isomer can be drawn.
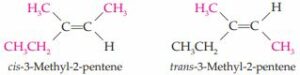
Each of the carbon atoms involved in the double bond is attached to two different groups. As a result, we can determine which is the cis isomer and which is the trans isomer based on the positions of the methyl groups relative to the double bond.