Question 13.2: Draw the structure of 3-ethyl-4-methylpent-2-ene using both ...
Draw the structure of 3-ethyl-4-methylpent-2-ene using both condensed and line structure.
ANALYSIS Identify the parent name (pent) and the location of the double bond and other substituents by numbering the carbons in the parent chain.
Learn more on how we answer questions.
STEP 1: The parent compound is a five-carbon chain with the double bond between C2 and C3.
\overset{1}{C} —\overset{2}{C}=\overset{3}{C}—\overset{4}{C}—\overset{5}{C} Pent-2-ene
STEP 2: Add the ethyl and methyl substituents on C3 and C4, and write in the additional hydrogen atoms so that each carbon atom has four bonds.

Using line structures we can draw it in the following ways:
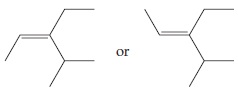
In this particular case either is correct. The two structures differ in the position of the CH_3 with respect to the CH_2CH_3; they are examples of cis–trans isomers (Section 13.3).