Question 10.1: Give names for the following compounds: Strategy Use the thr...
Give names for the following compounds:



Strategy Use the three-step procedure for naming substituted alkanes: (1) name the parent alkane, (2) number the carbons, and (3) name and number the substituent. (Consult Table 2.4 for parent alkane names.)
Setup (a) This is a five-carbon chain. We can number the carbons starting at either end because the Cl substituent will be located on carbon 3 either way:

(b) This may look like a substituted pentane, too, but the longest carbon chain in this molecule is seven carbons long.

Although Lewis structures appear to be flat and to contain 90° angles, the C atoms in this molecule are all sp³-hybridized (four electron domains around each) and there is free rotation about the C—C bonds [« Section 9.5]. Thus, the molecule can also be drawn as
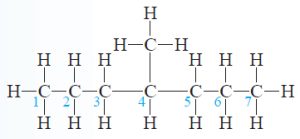
The substituent is a methyl group on carbon 4.
(c) This is a substituted hexane.

| TABLE 2.4 | Some Simple Acids | |
| Formula | Binary Compound Name | Acid Name |
| HF | Hydrogen fluoride | Hydrofluoric acid |
| HCl | Hydrogen chloride | Hydrochloric acid |
| HBr | Hydrogen bromide | Hydrobromic acid |
| HI | Hydrogen iodide | Hydroiodic acid |
| HCN* | Hydrogen cyanide | Hydrocyanic acid |
* Although HCN is not a binary compound, it is included in this table because it is similar chemically to HF, HCl, HBr, and HI.
Learn more on how we answer questions.
(a) 3-chloropentane, (b) 4-methylheptane, (c) 2-methylhexane