Question 12.2: Given the family of organic compounds to which the compound ...
Given the family of organic compounds to which the compound belongs, propose structures for compounds having the following chemical formulas.
(a) An amine having the formula C_2H_7N
(b) An alkyne having the formula C_3H_4
(c) An ether having the formula C_4H_ {10 }O
ANALYSIS Identify the functional group for each compound from Table 12.1. Once the atoms in this functional group are eliminated from the chemical formula, the remaining structure can be determined. (Remember that each carbon atom forms four bonds, nitrogen forms three bonds, oxygen forms two bonds, and hydrogen forms only one bond.)
| FAMILY NAME | FUNCTIONAL GROUP STRUCTURE* | SIMPLE EXAMPLE | NAME ENDING |
| Alkane | Contains only C—H and C—C single bonds | CH_3CH_3 Ethane | -ane |
| Alkene | -ene | ||
| Alkyne | -yne | ||
| Aromatic |  |
 Benzene Benzene |
None |
| Alkyl halide | CH_3 – Cl Methyl chloride | None | |
| Alcohol | CH_3 – OH Methyl alcohol (Methanol) | -ol | |
| Ether | CH_3 – O – CH_3 Dimethyl ether | None | |
| Amine | CH_3 – NH_2 Methylamine | -amine | |
| Aldehyde |  |
\begin{array}{r c}\underset{||}{O} \qquad \> \\ CH_3-C-H \end{array} Acetaldehyde (Ethanal) | -al |
| Ketone | 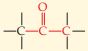 |
\begin{array}{r c}\underset{||}{O} \qquad \quad \> \\ CH_3-C-CH_3 \end{array} Acetone | -one |
| Carboxylic acid |  |
\begin{array}{r c}\underset{||}{O} \qquad \enspace \> \\ CH_3-C-OH \end{array} Acetic acid | -ic acid |
| Anhydride |  |
\begin{array}{r c}\underset{||}{O} \qquad \quad \> \underset{||}{O} \qquad \quad \> \\ CH_3-C-O-C-CH_3 \end{array} Acetic anhydride | None |
| Ester |  |
\begin{array}{r c} \underset{||}{O} \qquad \qquad \quad \> \\ CH_3-C-O-CH_3 \end{array} Methyl acetate | -ate |
| Amide |  |
\begin{array}{r c} \underset{||}{O} \qquad \quad \; \\ CH_3-C-NH_2 \end{array} Acetamide | -amide |
 |
|||
| *The bonds whose connections are not specified are assumed to be attached to carbon or hydrogen atoms in the rest of the molecule. | |||
Learn more on how we answer questions.
(a) Amines have a C – NH_2 group. Eliminating these atoms from the formula leaves 1 C atom and 5 H atoms. Since only the carbons are capable of forming more than one bond, the 2 C atoms must be bonded together. The remaining H atoms are then bonded to the carbons until each C has 4 bonds.

(b) The alkynes contain a C ≡ C bond. This leaves 1 C atom and 4 H atoms. Attach this C to one of the carbons in the triple bond, and then distribute the H atoms until each carbon has a full complement of four bonds.
\begin{array}{r c} \underset{|}{H} \qquad \qquad \qquad \> \\ H-C-C \equiv C-H \\ \overset{|}{H} \qquad \qquad \qquad \> \end{array}(c) The ethers contain a C — O — C group. Eliminating these atoms leaves 2 C atoms and 10 H atoms. The C atoms can be distributed on either end of the ether group, and the H atoms are then distributed until each carbon atom has a full complement of four bonds.
\begin{array}{r c} \underset{|}{H} \quad \underset{|}{H} \qquad \quad \; \underset{|}{H} \quad \underset{|}{H} \qquad \\ H-C-C-O-C-C-H \\ \overset{|}{H} \quad \overset{|}{H} \qquad \quad \; \overset{|}{H} \quad \overset{|}{H} \qquad \end{array} or \begin{array}{r c} \underset{|}{H} \quad \underset{|}{H} \quad \underset{|}{H} \qquad \quad \; \underset{|}{H} \qquad \\ H-C-C-C-O-C-H \\ \overset{|}{H} \quad \overset{|}{H} \quad \overset{|}{H} \qquad \quad \; \overset{|}{H} \qquad \end{array}