Question 5.SP.17: How many grams of aspirin are formed from 10.0 g of salicyli...
How many grams of aspirin are formed from 10.0 g of salicylic acid using the given balanced equation?
\begin{matrix} C_{7}H_{6}O_{3}(s)& +& C_{2}H_{4}O_{2}(I) & \longrightarrow & C_{9}H_{8}O_{4}(S) &+ & H_{2}O(I) \\ \text{salicylic acid} & & \text{acetic acid } & & \text{aspirin }\end{matrix}
Learn more on how we answer questions.
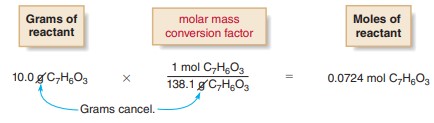
[1] Convert the number of grams of reactant to the number of moles of reactant using the
reactant’s molar mass.
• Use the molar mass of the reactant (C_{7}H_{6}O_{3}, molar mass 138.1 g/mol) to write a conversion factor. Multiply the number of grams of reactant by the conversion factor to give the number of moles of reactant.
[2] Convert the number of moles of reactant to the number of moles of product using a mole–mole conversion factor.
• Use the coefficients in the balanced chemical equation to write mole–mole conversion factors for the two compounds—one mole of salicylic acid (C_{7}H_{6}O_{3}) forms one mole of aspirin (C_{9}H_{8}O_{4}).
• Multiply the number of moles of reactant (salicylic acid) by the conversion factor to give the number of moles of product (aspirin).

[3] Convert the number of moles of product to the number of grams of product using the product’s molar mass.
• Use the molar mass of the product (C_{9}H_{8}O_{4}). , molar mass 180.2 g/mol) to write a conversion
factor. Multiply the number of moles of product (from step [2]) by the conversion factor to give the number of grams of product.
