Question 19.5: Predicting Spontaneous Redox Reactions and Sketching Electro...
Predicting Spontaneous Redox Reactions and Sketching Electrochemical Cells
Without calculating E^\circ _{cell}, predict whether each of the redox reactions is spontaneous. If the reaction is spontaneous as written, make a sketch of the electrochemical cell in which the reaction could occur. If the reaction is not spontaneous as written, write an equation for the spontaneous direction in which the reaction would occur and sketch the electrochemical cell in which the spontaneous reaction would occur. In your sketches, make sure to label the anode (which should be drawn on the left), the cathode, and the direction of electron flow.
(a) Fe(s) + Mg^{2+}(aq) \longrightarrow Fe^{2+}(aq) + Mg(s)
(b) Fe(s) + Pb^{2+}(aq) \longrightarrow Fe^{2+}(aq) + Pb(s)
Learn more on how we answer questions.
(a) Fe(s) + Mg^{2+}(aq) \longrightarrow Fe^{2+}(aq) + Mg(s)
This reaction involves the reduction of Mg^{2+}:
Mg^{2+}(aq) +2 e^- \longrightarrow Mg(s) E° = –2.37 V
and the oxidation of Fe:
Fe(s) \longrightarrow Fe^{2+}(aq) +2 e^- E° = –0.45 V
However, the magnesium half-reaction has the more negative electrode potential, and therefore, repels electrons more strongly and undergoes oxidation. The iron half-reaction has the more positive electrode potential, and therefore, attracts electrons more strongly and undergoes reduction. So the reaction as written is not spontaneous. (The reaction pairs the reduction of Mg^{2+} with the reverse of a half-reaction above it in Table 19.1—such pairings are not spontaneous.)
However, the reverse reaction is spontaneous:
Fe^{2+}(aq) +Mg(s) \longrightarrow Fe(s) + Mg^{2+}(aq)
The corresponding electrochemical cell is shown in Figure 19.9.
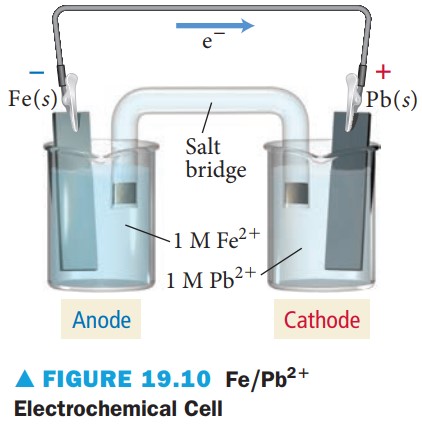
(b) Fe(s) + Pb^{2+}(aq) \longrightarrow Fe^{2+}(aq) + Pb(s)
This reaction involves the reduction of Pb^{2+}:
Pb^{2+}(aq) +2 e^- \longrightarrow Pb(s) E° = –0.13 V
and the oxidation of iron:
Fe(s) \longrightarrow Fe^{2+}(aq) +2 e^- E° = –0.45 V
The iron half-reaction has the more negative electrode potential, and therefore, repels electrons and undergoes oxidation. The lead half-reaction has the more positive electrode potential, and therefore, attracts electrons and undergoes reduction. Therefore, the reaction is spontaneous as written. (The reaction pairs the reduction of Pb^{2+} with the reverse of a half-reaction below it in Table 19.1—such pairings are always spontaneous.) The corresponding electrochemical cell is shown in Figure 19.10.
Table 19.1
| Standard Electrode Potentials at 25 °C | ||||
| Reduction Half-Reaction | E°(V) | |||
 |
F_2(g) + 2 e^- | \longrightarrow 2 F^-(aq) | 2.87 |  |
| H_2O_2(aq) + 2 H^+(aq) + 2 e^- | \longrightarrow 2 H_2O(l) | 1.78 | ||
| PbO_2(s) + 4 H^+(aq) + SO_4{}^{2-}(aq) + 2 e^- | \longrightarrow PbSO_4(s) + 2 H_2O(l) | 1.69 | ||
| MnO_4^-(aq) + 4 H^+(aq) + 3 e^- | \longrightarrow MnO_2(s) + 2 H_2O(l) | 1.68 | ||
| MnO_4{}^-(aq) + 8 H^+(aq) + 5 e^- | \longrightarrow Mn^{2 +}(aq) + 4 H_2O(l) | 1.51 | ||
| Au^{3 +}(aq) + 3 e^- | \longrightarrow Au(s) | 1.50 | ||
| PbO_2(s) + 4 H^+(aq) + 2 e^- | \longrightarrow Pb^{2 +}(aq) + 2 H_2O(l) | 1.46 | ||
| Cl_2(g) + 2 e^- | \longrightarrow 2 Cl^-(aq) | 1.36 | ||
| Cr_2O_7{}^{2 -}(aq) + 14 H^+(aq) + 6 e^- | \longrightarrow 2 Cr^{3 +}(aq) + 7 H_2O(l) | 1.33 | ||
| O_2(g) + 4 H^+(aq) + 4 e^- | \longrightarrow 2 H_2O(l) | 1.23 | ||
| MnO_2(s) + 4 H^+(aq) + 2 e^- | \longrightarrow Mn^{2 +}(aq) + 2 H_2O(l) | 1.21 | ||
| IO_3^-(aq) + 6 H^+(aq) + 5 e^- | \longrightarrow \frac{1}{2} I_2(aq) + 3 H_2O(l) | 1.20 | ||
| Br_2(l) + 2 e^- | \longrightarrow 2 Br^-(aq) | 1.09 | ||
| VO_2^{+}(aq) + 2 H^+(aq) + e^- | \longrightarrow VO^{2 +}(aq) + H_2O(l) | 1.00 | ||
| NO_3{}^{-}(aq) + 4 H^+(aq) + 3 e^- | \longrightarrow NO(g) + 2 H_2O(l) | 0.96 | ||
| ClO_2(g) + e^- | \longrightarrow ClO_2{}^-(aq) | 0.95 | ||
| Ag^+(aq) + e^- | \longrightarrow Ag(s) | 0.80 | ||
| Fe^{3 +}(aq) + e^- | \longrightarrow Fe^{2 +}(aq) | 0.77 | ||
| O_2(g) + 2 H^+(aq) + 2 e^- | \longrightarrow H_2O_2(aq) | 0.70 | ||
| MnO_4{}^-(aq) + e^- | \longrightarrow MnO_4{}^{2-}(aq) | 0.56 | ||
| I_2(s) + 2 e^- | \longrightarrow 2 I^-(aq) | 0.54 | ||
| Cu^+(aq) + e^- | \longrightarrow Cu(s) | 0.52 | ||
| O_2(g) + 2 H_2O(l) + 4 e^- | \longrightarrow 4 OH^-(aq) | 0.40 | ||
| Cu^{2+}(aq) + 2 e^- | \longrightarrow Cu(s) | 0.34 | ||
| SO_4{}^{2 -}(aq) + 4 H^+(aq) + 2 e^- | \longrightarrow H_2SO_3(aq) + H_2O(l) | 0.20 | ||
| Cu^{2+}(aq) + e^- | \longrightarrow Cu^+(aq) | 0.16 | ||
| Sn^{4+}(aq) + 2 e^- | \longrightarrow Sn^{2 +}(aq) | 0.15 | ||
| 2 H^+(aq) + 2 e^- | \longrightarrow H_2(g) | 0 | ||
| Fe^{3+}(aq) + 3 e^- | \longrightarrow Fe(s) | –0.036 | ||
| Pb^{2+}(aq) + 2 e^- | \longrightarrow Pb(s) | -0.13 | ||
| Sn^{2+}(aq) + 2 e^- | \longrightarrow Sn(s) | -0.14 | ||
| Ni^{2+}(aq) + 2 e^- | \longrightarrow Ni(s) | -0.23 | ||
| Cd^{2+}(aq) + 2 e^- | \longrightarrow Cd(s) | -0.40 | ||
| Fe^{2+}(aq) + 2 e^- | \longrightarrow Fe(s) | -0.45 | ||
| Cr^{3+}(aq) + e^- | \longrightarrow Cr^{2+}(aq) | -0.50 | ||
| Cr^{3+}(aq) + 3 e^- | \longrightarrow Cr(s) | -0.73 | ||
| Zn^{2+}(aq) + 2 e^- | \longrightarrow Zn(s) | -0.76 | ||
| 2 H_2O(l) + 2 e^- | \longrightarrow H_2(g) + 2 OH^-(aq) | -0.83 | ||
| Mn^{2+}(aq) + 2 e^- | \longrightarrow Mn(s) | -1.18 | ||
| Al^{3+}(aq) + 3 e^- | \longrightarrow Al(s) | -1.66 | ||
| Mg^{2+}(aq) + 2 e^- | \longrightarrow Mg(s) | -2.37 | ||
| Na^+(aq) + e^- | \longrightarrow Na(s) | -2.71 | ||
| Ca^{2+}(aq) + 2 e^- | \longrightarrow Ca(s) | -2.76 | ||
| Ba^{2+}(aq) + 2 e^- | \longrightarrow Ba(s) | -2.90 | ||
| K^+(aq) + e^- | \longrightarrow K(s) | -2.92 | ||
| Li^+(aq) + e^- | \longrightarrow Li(s) | -3.04 | ||