Question 19.3: Predicting the Type of Radioactive Decay Predict whether eac...
Predicting the Type of Radioactive Decay
Predict whether each nuclide is more likely to decay via beta decay or positron emission.
(a) Mg-28 (b) Mg-22 (c) Mo-102
Learn more on how we answer questions.
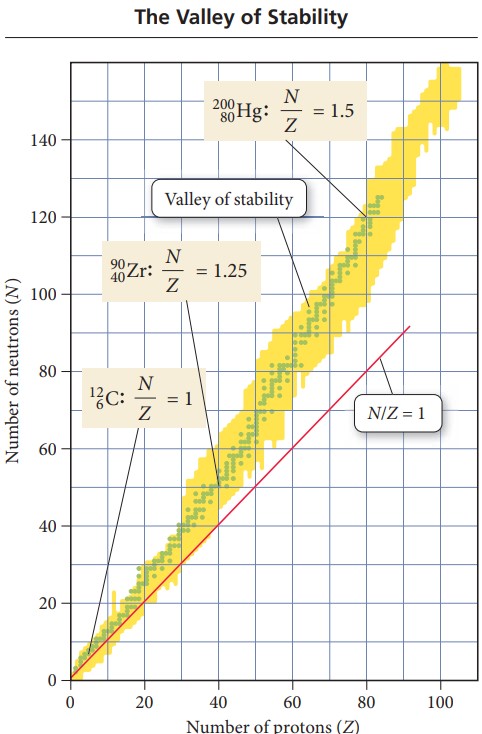
(a) Magnesium-28 has 16 neutrons and 12 protons, so N/Z = 1.33. However, for Z = 12, you can see in Figure 19.4 that stable nuclei should have an N/Z ratio of about 1. Alternatively, on the periodic table you can look up the atomic mass of magnesium, which is 24.31 amu; a magnesium nuclide with a mass number of 28 is too heavy to be stable because the N/Z ratio is too high. Therefore, Mg-28 undergoes beta decay, resulting in the conversion of a neutron to a proton.
(b) Magnesium-22 has 10 neutrons and 12 protons, so N/Z = 0.83 (too low). Alternatively, you can look up the atomic mass of magnesium, which is 24.31 amu; a magnesium nuclide with a mass number of 22 is too light (the N/Z ratio is too low). Therefore, Mg-22 undergoes positron emission, resulting in the conversion of a proton to a neutron.(Electron
capture would accomplish the same thing as positron emission, but in Mg-22, positron emission is the only decay mode observed.)
(c) Molybdenum-102 has 60 neutrons and 42 protons, so N/Z = 1.43. However, for Z = 42, you can see from Figure 19.4 that stable nuclei should have an N/Z ratio of about 1.3. Alternatively, you can look up the atomic mass of molybdenum, which is 95.94 amu; a molybdenum nuclide with a mass number of 102 is too heavy to be stable (the N/Z ratio is too high). Therefore, Mo-102 undergoes beta decay, resulting in the conversion of a neutron to a proton.
FiguRe 19.4 Stable and unstable Nuclei A plot of N (the number of neutrons) versus Z (the number of protons) for all known stable nuclei—represented by green dots on this graph—shows that these nuclei cluster together in a region known as the valley (or island) of stability. Nuclei with an N/Z ratio that is too high tend to undergo beta decay. Nuclei with an N/Zratio that is too low tend to undergo positron emission or electron capture.