Question 29.SIP.1: Pyruvic acid has the following structure:
Pyruvic acid has the following structure:

It is formed in the body from carbohydrate metabolism. In the muscle it is reduced to lactic acid in the course of exertion. The acid-dissociation constant for pyruvic acid is 3.2 × 10^{-3}.(a) Why does pyruvic acid have a higher acid-dissociation constant assuming a pH of about 7.4 and an acid concentration of 2 × 10^{-4} M? (c) What would you predict for the solubility properties of pyruvic acid? Explain. (d) What is the hybridization of each carbon atom in pyruvic acid? (e) Assuming H atoms as the reducing agent, write a balanced chemical equation for the reduction of pyruvic acid to lactic acid. (Although H atoms don’t exist as such in biochemical systems, biochemical reducing agents deliver hydrogen for such reductions.)
Learn more on how we answer questions.
(a) The acid ionization constant for pyruvic acid should be somewhat greater than that of acetic acid because the carbonyl function on the a-carbon atom exerts an electron-withdrawing effect on the carboxylic acid group. In the C—O—H bond system, the electrons are shifted from hydrogen, facilitating loss of the hydrogen as a proton.
(b) To determine the extent of ionization, we first set up the ionization equilibrium and equilibrium constant expression. Using HPv as the symbol for the acid, we have
\begin{gathered}HPv \leftrightharpoons H ^{+}+ Pv ^{-}\\K_{a}=\frac{\left[ H ^{+}\right]\left[ Pv ^{-}\right]}{[ HPv]}=3.2 \times 10^{-3}\end{gathered}
Let \left[ Pv ^{-}\right]=x. Then the concentration of undissociated acid is 2 × 10^{-4}-x . The concentration of [H^+] is fixed at 4.0 × 10^{-8} (the antilog of the pH value). Substituting, we obtain
3.2 \times 10^{-3}=\frac{\left[4.0 \times 10^{-8}\right][x]}{\left[2 \times 10^{-4}-x\right]}
Solving for x, we obtain x\left[3.2 \times 10^{-3}+4.0 \times 10^{-8}\right]=6.4 \times 10^{-7}
The second term in the brackets is negligible compared with the first, so x=\left[ Pv ^{-}\right]=6.4 \times 10^{-7}/ 3.2 \times 10^{-3}=2 \times 10^{-4}\,M. This is the initial concentration of acid, which means that essentially all the acid has dissociated. We might have expected this result because the acid is quite dilute and the acid-dissociation constant is fairly high.
(c) Pyruvic acid should be quite soluble in water because it has polar functional groups and a small hydrocarbon component. It is miscible with water, ethanol and diethyl ether.
(d) The methyl group carbon has sp² hybridization. The carbon carrying the carbonyl group has sp² hybridization because of the double bond to oxygen. Similarly, the carboxylic acid carbon is sp² hybridized.
(e) The balanced chemical equation for this reaction is
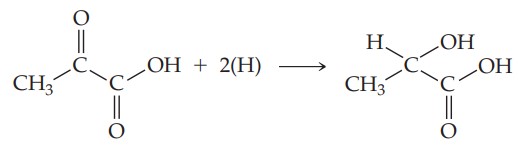
Essentially, the ketonic functional group has been reduced to an alcohol.