Question 9.SE.2: Use the VSEPR model to predict the molecular geometry of (a)...
Use the VSEPR model to predict the molecular geometry of (a) SF_4, IF_5.
Learn more on how we answer questions.
Analyze The molecules are of the AB_n type with a central p-block atom.
Plan We first draw Lewis structures and then use the VSEPR model to determine the electron-domain geometry and molecular geometry.
Solve
(a) The Lewis structure for SF_4 is:

The sulfur has five electron domains around it: four from the S—F bonds and one from the nonbonding pair. Each domain points toward a vertex of a trigonal bipyramid. The domain from the nonbonding pair will point toward an equatorial position. The four bonds point toward the remaining four positions, resulting in a molecular geometry that is described as seesaw-shaped:

Comment The experimentally observed structure is shown on the right. We can infer that the nonbonding electron domain occupies an equatorial position, as predicted. The axial and equatorial S—F bonds are slightly bent away from the nonbonding domain, suggesting that the bonding domains are “pushed” by the nonbonding domain, which exerts a greater repulsion (Figure 9.7).

Solve
(b) The Lewis structure of IF_5 is:

The iodine has six electron domains around it, one of which is nonbonding. The electron-domain geometry is therefore octahedral, with one position occupied by the nonbonding pair, and the molecular geometry is square pyramidal (Table 9.3):
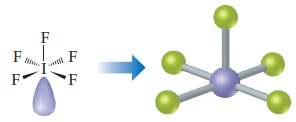
Comment Because the nonbonding domain is larger than the bonding domains, we predict that the four F atoms in the base of the pyramid will be tipped up slightly toward the top F atom. Experimentally, we find that the angle between the base atoms and the top F atom is 82°, smaller than the ideal 90° angle of an octahedron.
| TABLE 9.3 Electron-Domain and Molecular Geometries for Five and Six Electron Domains around a Central Atom | |||||
| Number of Electron Domains |
Electron-Domain Geometry |
Bonding Domains |
Nonbonding Domains |
Molecular Geometry |
Example |
| 5 |  |
5 | 0 |  |
PCl_5 |
| 4 | 1 |  |
SF_4 | ||
| 3 | 2 |  |
ClF_3 | ||
| 2 | 3 |  |
XeF_2 | ||
| 6 |  |
6 | 0 |  |
SF_6 |
| 5 | 1 |  |
BrF_5 | ||
| 4 | 2 |  |
XeF_4 | ||
