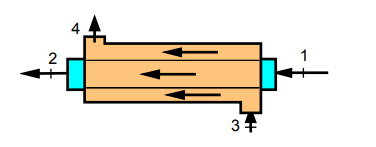
A coflowing (same direction) heat exchanger has one line with 0.5 lbm/s oxygen at 68 F and 30 psia entering and the other line has 1.2 lbm/s nitrogen at 20 psia and 900 R entering. The heat exchanger is long enough so that the two flows exit at the same temperature. Use constant heat capacities and find the exit temperature and the second law efficiency for the heat exchanger assuming ambient at 68 F.