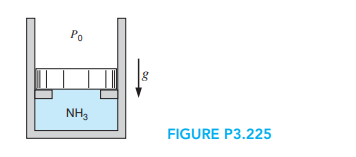
A piston/cylinder contains 2 lbm of water at 70 F with a volume of 0.1 ft ^{3}, shown in Fig. P3.225. Initially the piston rests on some stops with the top surface open to the atmosphere, Po, so a pressure of 40 lbf/in .^{2} is required to lift it. To what temperature should the water be heated to lift the piston? If it is heated to saturated vapor find the final temperature, volume, and the heat transfer.
Question 3.301E: A piston/cylinder contains 2 lbm of water at 70 F with a vol...
The Blue Check Mark means that this solution has been answered and checked by an expert. This guarantees that the final answer is accurate.
Learn more on how we answer questions.
Learn more on how we answer questions.
C.V. Water. This is a control mass.
m _{2}= m _{1}= m ; \quad m \left( u _{2}- u _{1}\right)={ }_{1} Q _{2}-{ }_{1} W _{2}State 1: 20 C, v _{1}= V / m =0.1 / 2=0.05 ft ^{3} / lbm
x =(0.05-0.01605) / 867.579=0.0003913
u _{1}=38.09+0.0003913 \times 995.64=38.13 Btu / lbm
To find state 2 check on state 1a:
P = 40 psia, v = v _{1}=0.05 ft ^{3} / lbm
Table F.7.1: v _{ f }< v < v _{ g }=10.501, x _{1 a }>0
State 2 is saturated vapor at 40 psia as state 1a is two-phase. T _{2}=267.3 F
v _{2}= vg =10.501 ft ^{3} / lbm , V _{2}= m v _{2}=21.0 ft ^{3}, u _{2}= ug =1092.27 Btu / lbm
Pressure is constant as volume increase beyond initial volume.
{ }_{1} W _{2}=\int P d V = P _{ lift }\left( V _{2}- V _{1}\right)=40(21.0-0.1) \times 144 / 778=154.75 Btu
{ }_{1} Q _{2}= m \left( u _{2}- u _{1}\right)+{ }_{1} W _{2}=2(1092.27-38.13)+154.75=2263 Btu



Related Answered Questions
C.V. A + B . Then { }_{1} Q _{2}=\emptys...
C.V. The 1 lbm water.
Continuty: m _{2}=...
C.V. The mass of R-134a. Properties in Table F.10....
State 1: T _{1}, \quad v _{1}= V / m =\fra...
\dot{ Q }= k A \frac{\Delta T }{\Delta x }[...
Steady state conduction through a single layer boa...
One dimensional heat transfer by conduction, we do...
\dot{ W }= F V =300 lbf \times 40( mi / h...