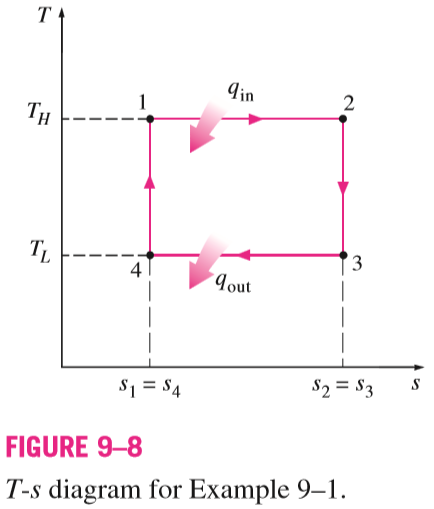
Derivation of the Efficiency of the Carnot Cycle
Show that the thermal efficiency of a Carnot cycle operating between the temperature limits of T_{H} \text { and } T_{L} is solely a function of these two temperatures and is given by Eq. 9–2.
\eta_{ th , Carnot }=1-\frac{T_{L}}{T_{H}}