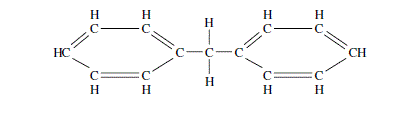
Estimate the critical constants for diphenylmethane using Lydersen’s method; normal boiling point 537.5 K, molecular mass 168.2, structural formula:
Question 8.14: Estimate the critical constants for diphenylmethane using Ly...
The Blue Check Mark means that this solution has been answered and checked by an expert. This guarantees that the final answer is accurate.
Learn more on how we answer questions.
Learn more on how we answer questions.
| Group | No. of | Total contribution | ||
| \Delta T | \Delta P | \Delta V | ||
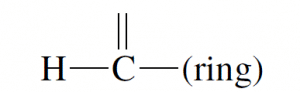 |
10 | 0.11 | 1.54 | 0.37 |
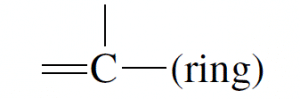 |
2 | 0.022 | 0.308 | 0.072 |
| – CH _{2}- | 1 | 0.02 | 0.227 | 0.055 |
| \Sigma 0.152 | 2.075 | 0.497 | ||
T_{c}=\frac{537.5}{\left(0.567+0.152-0.152^{2}\right)}=\underline{\underline{772 k }}
experimental value 767 K,
P_{c}=\frac{168.2}{(0.34+2.075)^{2}}=28.8 atm
experimental value 28.2 atm,
V_{c}=0.04+0.497=0.537 m ^{3} / kmol
Related Answered Questions
Diffusion volumes from Table 8.5; methanol:
...
Viscosity of ethanol at 20^{\circ} C , 1.2 ...
Calculation of parachor, CH _{3} OH...
Use the binary Wilson A values given by Hirata (19...
Matrix of coefficients
j
1
2
3
4
i...
\text { Temperature increment } 80-20=60^{\...
Toluene
Table 8.1. Contributions for ca...
\text { Density at } 30^{\circ} C =875 kg /...
Viscosity = 0.0134 mNs / m ^{2}
Spe...