Estimate the specific heat capacity of urea, CH _{4} N _{2} O .
Question 8.6: Estimate the specific heat capacity of urea, CH4N2O.
The Blue Check Mark means that this solution has been answered and checked by an expert. This guarantees that the final answer is accurate.
Learn more on how we answer questions.
Learn more on how we answer questions.
| Element | mol. mass | Heat capacity |
| C | 12 | 7.5 = 7.5 |
| H | 4 | 4 \times 9.6=38.4 |
| N | 28 | 2 \times 26.0=52.0 |
| O | 16 | 16.7 = 16.7 |
| 60 | 114.6 J / mol ^{\circ} C |
\text { Specific heat capacity }=\frac{114.6}{60}=1.91 J / g ^{\circ} C \left( kJ / kg ^{\circ} C \right)
Experimental value 1.34 kJ / kg ^{\circ} C.
Kopp’s rule does not take into account the arrangement of the atoms in the molecule, and, at best, gives only very approximate, “ball-park” values.
For organic liquids, the group contribution method proposed by Chueh and Swanson (1973a,b) will give accurate predictions. The contributions to be assigned to each molecular group are given in Table 8.3 and the method illustrated in Examples 8.7 and 8.8. Liquid specific heats do not vary much with temperature, at temperatures well below the critical temperature (reduced temperature <0.7).
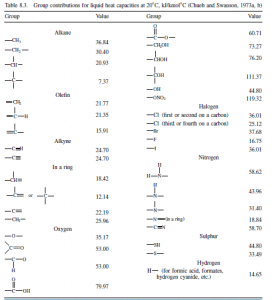
Add 18.84 for any carbon group which fulfils the following criterion: a carbon group which is joined by a single bond to a carbon group connected by a double or triple bond with a third carbon group. In some cases a carbon group fulfils the above criterion in more ways than one; 18.84 should be added each time the group fulfils the criterion.
Exceptions to the above 18.84 rule:
The specific heats of liquid mixtures can be estimated, with sufficient accuracy for most technical calculations, by taking heat capacities of the components as additive.
For dilute aqueous solutions it is usually sufficient to take the specific heat of the solution as that of water.
Related Answered Questions
Diffusion volumes from Table 8.5; methanol:
...
Viscosity of ethanol at 20^{\circ} C , 1.2 ...
Calculation of parachor, CH _{3} OH...
Group
No. of
Total contribution
\Delta T[...
Use the binary Wilson A values given by Hirata (19...
Matrix of coefficients
j
1
2
3
4
i...
\text { Temperature increment } 80-20=60^{\...
Toluene
Table 8.1. Contributions for ca...
\text { Density at } 30^{\circ} C =875 kg /...