Question 8.2: Estimate the viscosity of toluene at 20ºC.
Learn more on how we answer questions.
Toluene
| Table 8.1. Contributions for calculating the viscosity constant I in Souders’ equation | |||||||
| Atom | H | O | C | N | Cl | Br | I |
| Contribution | +2.7 | +29.7 | +50.2 | +37.0 | +60 | +79 | +110 |
| Contributions of groups and bonds | |||||||
| Double bond | -15.5 | 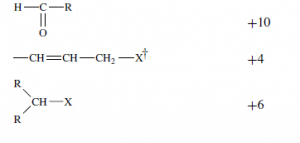 |
|||||
| Five-member ring | -24 | ||||||
| Six-member ring | -21 | ||||||
| Side groups on a | |||||||
| six-member ring: | |||||||
| Molecular weight < 17 | -9 | ||||||
| Molecular weight > 16 | -17 | ||||||
| Ortho or para position | +3 | ||||||
| Meta position | -1 | ||||||
 |
OH | +57.1 | |||||
| COO | +90 | ||||||
| COOH | +104.4 | ||||||
| NO _{2} | +80 | ||||||
\dagger X \text { is a negative group }.
Contributions from Table 8.1:
7 carbon atoms 7 \times 50.2=351.4
8 hydrogen atoms 8 \times 27=216
3 double bonds 3(-15.5) = -46.5
1 six-membered ring -21.1
1 side group
Total, I = 296.4
Density at 20^{\circ} C = 866 kg / m ^{3}
Molecular weight 92
\log (\log 10 \mu)=\frac{296.4 \times 866 \times 10^{-3}}{00}-2.9=-0.11
\log 10 \mu=0.776
\mu=0.597, \text { rounded }=0.6 mNs / m ^{2}
experimental value, 0.6 cp =0.6 mNs / m ^{2}
Author’s note: the fit obtained in this example is rather fortuitous, the usual accuracy of the method for organic liquids is around \pm 10 per cent.
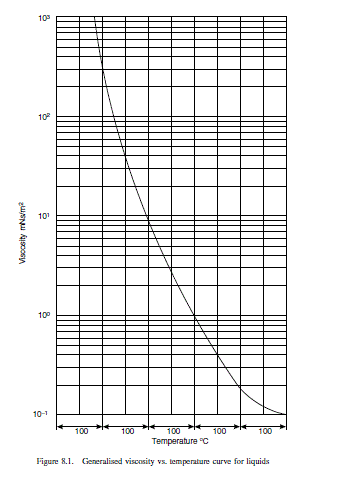
Variation with temperature
If the viscosity is known at a particular temperature, the value at another temperature can be estimated with reasonable accuracy (within \pm 20 per cent) by using the generalised
plot of Lewis and Squires (1934), Figure 8.1. The scale of the temperature ordinate is obtained by plotting the known value, as illustrated in Example 8.3.