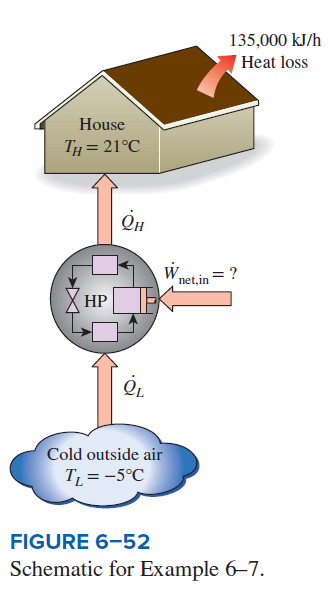
Heating a House with a Carnot Heat Pump
A heat pump is to be used to heat a house during the winter, as shown in Fig. 6–52. The house is to be maintained at 21°C at all times. The house is estimated to be losing heat at a rate of 135,000 kJ/h when the outside temperature drops to –5°C. Determine the minimum power required to drive this heat pump.