(a) From Equation 2.197:
-\lambda \psi_{j+1}+\left(2 \lambda+V_{j}\right) \psi_{j}-\lambda \psi_{j-1}=E \psi_{j}, \quad \text { where } \quad \lambda \equiv \frac{\hbar^{2}}{2 m(\Delta x)^{2}} . (2.197).
N=1: j=1:-\lambda \cancel{\psi_{2}}+(2 \lambda) \psi_{1}-\lambda \cancel{\psi_{0}}=E \psi_{1} \quad \Rightarrow \quad H =(2 \lambda) .
N=2:\left\{\begin{array}{l} j=1:-\lambda \psi_{2}+(2 \lambda) \psi_{1}-\lambda \cancel{\psi_{0}}=E \psi_{1} \\ j=2:-\lambda \cancel{\psi_{3}}+(2 \lambda) \psi_{2}-\lambda \psi_{1}=E \psi_{2} \end{array} \quad \Rightarrow\right. H =\left(\begin{array}{cc} 2 \lambda & -\lambda \\ -\lambda & 2 \lambda \end{array}\right) .
N=3:\left\{\begin{array}{l} j=1:-\lambda \psi_{2}+(2 \lambda) \psi_{1}-\lambda \cancel{\psi_{0}}=E \psi_{1} \\ j=2:-\lambda \psi_{3}+(2 \lambda) \psi_{2}-\lambda \psi_{1}=E \psi_{2} \\ j=3:-\lambda \cancel{\psi_{4}}+(2 \lambda) \psi_{3}-\lambda \psi_{2}=E \psi_{2} \end{array} \Rightarrow\right. H =\left(\begin{array}{ccc} 2 \lambda & -\lambda & 0 \\ -\lambda & 2 \lambda & -\lambda \\ 0 & -\lambda & 2 \lambda \end{array}\right) .
(b) Denote the eigenvalues by \tilde{E}_{n} :
N=1: \tilde{E}_{1}=2 \lambda=\frac{2 \hbar^{2}}{2 m(a / 2)^{2}}=\frac{8 \hbar^{2}}{2 m a^{2}} . The exact energies are E_{n}=\frac{n^{2} \pi^{2} \hbar^{2}}{2 m a^{2}}, \text { so } E_{1}=\frac{\pi^{2} \hbar^{2}}{2 m a^{2}} ; the agreement is not too bad: 8 \approx \pi^{2}=9.87 .
N = 2 :
\operatorname{det}\left(\begin{array}{cc} 2 \lambda-\tilde{E} & -\lambda \\ -\lambda & 2 \lambda-\tilde{E} \end{array}\right)=0 \Rightarrow(2 \lambda- \tilde{E})^{2}-\lambda^{2}=0 \Rightarrow 2 \lambda-\tilde{E}=\pm \lambda .
\tilde{E}_{1}=2 \lambda-\lambda=\lambda=\frac{\hbar^{2}}{2 m(a / 3)^{2}}=\frac{9 \hbar^{2}}{2 m a^{2}} . This is better: 9 is closer to π² than 8 was.
\tilde{E}_{2}=2 \lambda+\lambda=3 \lambda=\frac{3 \hbar^{2}}{2 m(a / 3)^{2}}=\frac{27 \hbar^{2}}{2 m a^{2}} . The exact answer has 4π² = 39.5 instead of 27.
N = 3 :
\operatorname{det}\left(\begin{array}{ccc} 2 \lambda-\tilde{E} & -\lambda & 0 \\ -\lambda & 2 \lambda-\tilde{E} & -\lambda \\ 0 & -\lambda & 2 \lambda-\tilde{E}<br />
\end{array}\right)=0 \Rightarrow(2 \lambda-\tilde{E})^{3}-2 \lambda^{2}(2 \lambda-\tilde{E})=0 \Rightarrow 2 \lambda-\tilde{E}=0 \text { or } 2 \lambda-\tilde{E}=\pm \sqrt{2} \lambda .
\tilde{E}_{1}=2 \lambda-\sqrt{2} \lambda=\lambda(2-\sqrt{2})=\frac{(2-\sqrt{2}) \hbar^{2}}{2 m(a / 4)^{2}}=\frac{16(2-\sqrt{2}) \hbar^{2}}{2 m a^{2}} . This is better yet: 16(2-\sqrt{2})=9.37 .
\tilde{E}_{2}=2 \lambda=\frac{2 \hbar^{2}}{2 m(a / 4)^{2}}=\frac{32 \hbar^{2}}{2 m a^{2}} . Improving: the exact answer is 4π² = 39.5 instead of 32.
\tilde{E}_{3}=2 \lambda+\sqrt{2} \lambda=\lambda(2+\sqrt{2})=\frac{(2+\sqrt{2}) \hbar^{2}}{2 m(a / 4)^{2}}=\frac{16(2+\sqrt{2}) \hbar^{2}}{2 m a^{2}} ; 16(2+\sqrt{2})=54.6 \approx 9 \pi^{2}=88.8 .
(c)
h = Table [If [i == j, 2 λ, 0], \{i, 10\},\{j, 10\} ].
k = Table [If [i == j+1, – λ, 0], \{i, 10\},\{j, 10\} ].
m = Table [If [i == j-1, – λ, 0], \{i, 10\},\{j, 10\} ].
p = Table [h [ [i , j]] + k [[i,j]] + m [[i,j]] , \{i, 10\},\{j, 10\} ].
P = MatrixForm[%]
\left(\begin{array}{cccccccccc} 2 \lambda & -\lambda & 0 & 0 & 0 & 0 & 0 & 0 & 0 & 0 \\ -\lambda & 2 \lambda & -\lambda & 0 & 0 & 0 & 0 & 0 & 0 & 0 \\ 0 & -\lambda & 2 \lambda & -\lambda & 0 & 0 & 0 & 0 & 0 & 0 \\ 0 & 0 & -\lambda & 2 \lambda & -\lambda & 0 & 0 & 0 & 0 & 0 \\ 0 & 0 & 0 & -\lambda & 2 \lambda & -\lambda & 0 & 0 & 0 & 0 \\ 0 & 0 & 0 & 0 & -\lambda & 2 \lambda & -\lambda & 0 & 0 & 0 \\ 0 & 0 & 0 & 0 & 0 & -\lambda & 2 \lambda & -\lambda & 0 & 0 \\ 0 & 0 & 0 & 0 & 0 & 0 & -\lambda & 2 \lambda & -\lambda & 0 \\ 0 & 0 & 0 & 0 & 0 & 0 & 0 & -\lambda & 2 \lambda & -\lambda \\ 0 & 0 & 0 & 0 & 0 & 0 & 0 & 0 & -\lambda & 2 \lambda \end{array}\right) .
λ=1
1
EIG = Eigenvalues [N[p]].
\left\{83.91899, 3.68251, 3.30972, 2.83083, 2.28463,1.71537, 1.16917, 0.690279, 0.317493, 0.0810141\right\} .
To get the eigenvalues, multiply by \lambda=\frac{\hbar^{2}}{2 m(a / 11)^{2}}=\left(\frac{121}{\pi^{2}}\right) E_{1} :
121 * EIG / (π^2).
\left\{848.0462, 45.147, 40.5767, 34.7056, 28.0092, 21.0302, 14.3339, 8.46272, 3.89242, 0.993221\right\} .
Thus the lowest five eigenvalues, in units of E_1 , are 0.9932, 3.8924, 8.4627, 14.3339, 21.0302, as compared to the exact values 1, 4, 9, 16, 25. Doing the same for N = 100:
h = Table [If [i == j, 2 λ, 0], \{i, 100\},\{j, 100\} ].
k = Table [If [i == j+1, – λ, 0], \{i, 100\},\{j, 100\} ].
m = Table [If [i == j-1, – λ, 0], \{i, 100\},\{j, 100\} ].
p = Table [h [ [i , j]] + k [[i,j]] + m [[i,j]] , \{i, 100\},\{j, 100\} ].
λ=1
1
EIG = Eigenvalues[N[p]]
10201 * EIG/(π^2).
{ 4133.31 , 4130.31 , 4125.32 , 4118.33 , 4109.36 , 4098.41 , 4085.5 , 4070.64 , 4053.84 , 4035.11 ,
4014.49 , 3991.97 , 3967.6 , 3941.39 , 3913.36 , 3883.55 , 3851.98 , 3818.69 , 3783.7,3747.04,
3708.77,3668.9,3627.49,3584.57,3540.18,3494.36, 3447.16,3398.63,3348.81,3297.75,
3245.5,3192.11,3137.63,3082.12,3025.62,2968.2,2909.9,2850.79,2790.92,2730.35,
2669.14,2607.35,2545.03,2482.25,2419.07,2355.55,2291.76,2227.74,2163.57,2099.3,
2035.011970.74,1906.57,1842.55,1778.75,1715.24,1652.06,1589.28,1526.96,1465.17,1403.96,
1343.39,1283.52,1224.41,1166.11,1108.69,1052.19, 996.679,942.201,888.81,836.559,
785.499,735.679,687.147,639.95,594.133,549.742,506.819,465.405,425.541,387.265,
350.614,315.624,282.328,250.76,220.948,192.922,166.71,142.336,119.824,99.1963,
80.4724,63.6704,48.8067,35.8956,24.9496,15.9794,8.99347,3.99871,0.999919 }.
This time the lowest five eigenvalues are 0.9999, 3.9987, 8.9934, 15.9794, 24.9496.
(d) Always, \psi_{0}=\psi_{N+1}=0 ;might as well set λ=1 for the last part.
N = 1 : 2 \psi_{1}=\tilde{E} \psi_{1}, \tilde{E}_{1}=2 Up to normalization:
N=2:\left(\begin{array}{cc} 2 & -1 \\ -1 & 2 \end{array}\right)\left(\begin{array}{l} \psi_{1} \\ \psi_{2} \end{array}\right)=\tilde{E}\left(\begin{array}{l} \psi_{1} \\ \psi_{2} \end{array}\right) \Rightarrow 2 \psi_{1}-\psi_{2}=\tilde{E} \psi_{1} \text { and }-\psi_{1}+2 \psi_{2}=\tilde{E} \psi_{2} .
n=1: \tilde{E}_{1}=1 \Rightarrow 2 \psi_{1}-\psi_{2}=\psi_{1} \Rightarrow \psi_{2}=\psi_{1} .
n=2: \tilde{E}_{2}=3 \Rightarrow 2 \psi_{1}-\psi_{2}=3 \psi_{1} \Rightarrow \psi_{2}=-\psi_{1} .
N=3:\left(\begin{array}{ccc} 2 & -1 & 0 \\ -1 & 2 & -1 \\ 0 & -1 & 2 \end{array}\right)\left(\begin{array}{l} \psi_{1} \\ \psi_{2} \\ \psi_{3} \end{array}\right)=\tilde{E}\left(\begin{array}{l} \psi_{1} \\ \psi_{2} \\ \psi_{3} \end{array}\right) \Rightarrow 2 \psi_{1}-\psi_{2}=\tilde{E} \psi_{1},-\psi_{1}+2 \psi_{2}-\psi_{3}=\tilde{E} \psi_{2},-\psi_{2}+2 \psi_{3}=\tilde{E} \psi_{3} .
n=1: \tilde{E}_{1}=2-\sqrt{2} \Rightarrow 2 \psi_{1}-\psi_{2}=(2-\sqrt{2}) \psi_{1} \Rightarrow \psi_{2}=\sqrt{2} \psi_{1} ;
-\psi_{1}+2 \psi_{2}-\psi_{3}=(2-\sqrt{2}) \psi_{2} \Rightarrow \psi_{1}+\psi_{3}=\sqrt{2} \psi_{2}=2 \psi_{1} \Rightarrow \psi_{3}=\psi_{1} .
n=2: \tilde{E}_{2}=2 \Rightarrow 2 \psi_{1}-\psi_{2}=2 \psi_{1} \Rightarrow \psi_{2}=0 ;-\psi_{1}+2 \psi_{2}-\psi_{3}=2 \psi_{2} \Rightarrow \psi_{3}=-\psi_{1} .
n=3: \tilde{E}_{3}=2+\sqrt{2} \Rightarrow 2 \psi_{1}-\psi_{2}=(2+\sqrt{2}) \psi_{1} \Rightarrow \psi_{2}=-\sqrt{2} \psi_{1} ;
-\psi_{1}+2 \psi_{2}-\psi_{3}=(2+\sqrt{2}) \psi_{2} \Rightarrow \psi_{1}+\psi_{3}=-\sqrt{2} \psi_{2}=2 \psi_{1} \Rightarrow \psi_{3}=\psi_{1} .
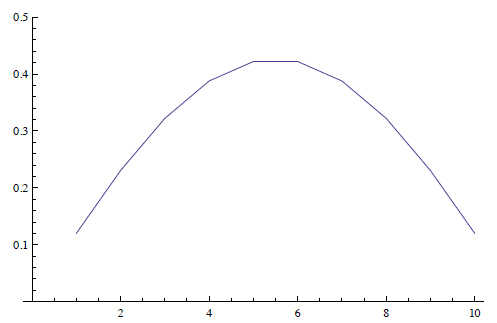
For N = 10 we get
EVE = Eigenvectors [N[p]]
ListLinePlot [EVE[[10]], PlotRange → \left\{0,0.5\right\} ].
ListLinePlot [EVE[[9]], PlotRange → \left\{-0.5,0.5\right\} ].
ListLinePlot [EVE[[8]], PlotRange → \left\{-0.5,0.5\right\} ].
and for N = 100
EVE = Eigenvectors [N[p]]
ListLinePlot [EVE[[100]], PlotRange → \left\{0,0.2\right\} ].
ListLinePlot [EVE[[99]], PlotRange → \left\{-0.2,0.2\right\} ].
ListLinePlot [EVE[[98]], PlotRange → \left\{-0.2,0.2\right\} ].