Test of the UNIFAC Model
Compare the UNIFAC predictions for the activity coefficients of the benzene–2,2,4-trimethyl pentane mixture with the experimental data given in Illustration 9.5-1.
Test of the UNIFAC Model
Compare the UNIFAC predictions for the activity coefficients of the benzene–2,2,4-trimethyl pentane mixture with the experimental data given in Illustration 9.5-1.
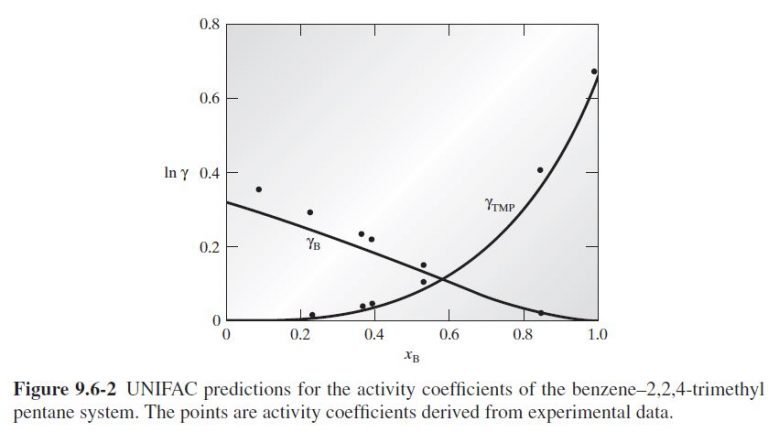
Benzene consists of six aromatic CH groups (i.e., subgroup 10 of Table 9.5-2), whereas 2,2,4-trimethyl pentane contains five CH _{3} groups (subgroup 1), one CH _{2} group (subgroup 2), one CH group (subgroup 3), and one C group (subgroup 4). Using the UNIFAC program of Aspen Plus^R with the folder Aspen Illustrations>Chapter 9>9.6-2 on the Wiley website for this book, the results plotted in Fig. 9.6-2 are obtained.
It is clear from this figure that, for this simple system, the UNIFAC predictions are good—much better than the regular solution predictions of Fig. 9.6-1. Although the UNIFAC predictions for all systems are not always as good as for the benzene–2,2,4-trimethyl pentane system, UNIFAC with its recent improvements is the best activity coefficient predictionmethod currently available. [The Excel file Illustration 9.6-2 in Appendix C on the website for this book gives the results.]
| Table 9.5-2 The Group Volume and Surface Area Parameters, R and Q, for Use with the UNIQUAC and UNIFAC Models* | ||||
| Main Group | Subgroup | R | Q | Example Assignments |
| CH _{2} | CH _{3} | 0.6325 | 1.0608 | n-Hexane: 4 CH _{2}, 2 CH _{3} |
| CH _{2} | 0.6325 | 0.7081 | n-Heptane:5 CH _{2}, 2 CH _{3} | |
| CH | 0.6325 | 0.3554 | 2-Methylpropane:1 CH , 3 CH _{3} | |
| C | 0.6325 | 0 | Neopentane: 1 C , 4 CH _{3} | |
| C=C | CH _{2}= CH | 1.2832 | 1.6016 | 1-Hexene: 1 CH _{2}= CH , 3 CH _{2}, 1 CH _{3} |
| CH=CH | 1.2832 | 1.2489 | 2-Hexene: 1 CH = CH , 2 CH _{3}, 2 CH _{2} | |
| CH _{2}= C | 1.2832 | 1.2489 | 2-Methyl-1-butene: 1 CH _{2}= C , 1 CH _{2}, 2 CH _{3} | |
| CH=C | 1.2832 | 0.8962 | 2-Methyl-2-butene: 1 CH = C , 2 CH _{3} | |
| C=C | 1.2832 | 0.4582 | 12,3-Dimethyl-2-butene: 1 C = C , 4 CH _{3} | |
| ACH | ACH | 0.3763 | 0.4321 | Benzene: 6 ACH |
| AC | 0.3763 | 0.2113 | Styrene: 1 CH _{2}= C , 5 ACH _{2}, 1 ACH | |
| ACCH _{2} | ACCH _{3} | 0.91 | 0.949 | Toluene: 5 ACH _{1}, 1ACCH _{2}, 1 CH _{3} |
| ACCH _{2} | 0.91 | 0.7962 | Ethylbenzene: 5 ACH , 1 ACCH _{2}, 1 CH _{3} | |
| ACCH | 0.91 | 0.3769 | Isopropylbenzene: 5 ACH , 1 ACCH , 2 CH _{3} | |
| OH | OH(p) | 1.2302 | 0.8927 | 1-Propanol: 1 OH ( p ), 1 CH _{3}, 2 CH _{2} |
| OH(s) | 1.063 | 0.8663 | 2-Propanol: 1 OH ( s ), 2 CH _{3}, 1 CH | |
| OH(t) | 0.6895 | 0.8345 | tert-Butanol: 1 OH ( t ), 3 CH _{3}, 1 C | |
| CH _{3} OH | CH _{3} OH | 0.8585 | 0.9938 | Methanol |
| Water | H _{2} O | 1.7334 | 2.4561 | Water |
| ACOH | ACOH | 1.08 | 0.975 | Phenol: 1 ACOH, 5 ACH |
| CH _{2} CO | CH _{3} CO | 1.7048 | 1.67 | 2-Butanone: 1 CH _{3} CO , 1 CH _{3}, 1 CH _{2} |
| CH _{2} CO | 1.7048 | 1.5542 | 2-Pentanone: 1 CH _{2} CO , 2 CH _{3}, 1 CH _{2} | |
| CHO | CHO | 0.7173 | 0.771 | Propionic aldehyde: 1 CHO , 1 CH _{3}, 1 CH _{2} |
| CCOO | CH _{3} COO | 1.27 | 1.6286 | Butyl acetate: 1 CH _{3} COO , 1 CH _{3}, 3 CH _{2} |
| CH _{2} COO | 1.27 | 1.4228 | Methyl propionate: 1 HCOO , 1 CH _{3}, 1 CH _{2} | |
| HCOO | HCOO | 1.9 | 1.8 | Ethyl formate: 1 HCOO , 1 CH _{3}, 1 CH _{2} |
| CH _{2} O | CH _{3} O | 1.1434 | 1.6022 | Dimethyl ether: 1 CH _{3} CO , 1 CH _{3} |
| CH _{2} O | 1.1434 | 1.2495 | Diethyl ether: 1 CH _{2} O , 2 CH _{3}, 1 CH _{2} | |
| CNH _{2} | CH _{3} NH _{2} | 1.1434 | 0.8968 | Diisopropyl ether: 1 CHO , 4 CH _{3}, 1 CH |
| CH _{2} NH _{2} | 1.6607 | 1.6904 | Methylamine: CH _{3} NH _{2} | |
| CHNH _{2} | 1.6607 | 1.3377 | Ethylamine: 1 CH _{2} NH _{2}, 1 CH _{3} | |
| CNH _{2} | 1.6607 | 0.985 | Isopropylamine: 1 CHNH _{2}, 2 CH _{3} | |
| CNH | CH _{3} NH | 1.6607 | 0.985 | tert-Butylamine: 1 CNH _{2}, 3 CH _{3} |
| CH _{2} NH | 1.368 | 1.4332 | Dimethylamine: CH _{3} NH , 1 CH _{3} | |
| CHNH | 1.368 | 1.0805 | Diethylamine: 1 CH _{2} NH , 2 CH _{3}, 1 CH _{2} | |
| ( C )_{3} N | CH _{3} N | 1.368 | 0.7278 | Diisopropylamine: 1 CHNH , 4 CH _{3}, 1 CH |
| CH _{2} N | 1.0746 | 1.176 | Trimethylamine: 1 CH _{3} N , 2 CH _{3} | |
| ACNH _{2} | ACNH _{2} | 1.0746 | 0.824 | Triethylamine: 1 CH _{3} N , 2 CH _{3} |
| Pyridines | AC _{2} H _{2} N | 1.1849 | 0.8067 | Aniline: 1 ACNH _{2}, 5 ACH |
| AC _{2} HN | 1.4578 | 0.9022 | Pyridine: 1 AC _{2} H _{2} N , 3 ACH | |
| AC _{2} N | 1.2393 | 0.633 | 2-Methylpyridine: 1 AC _{2} HN , 3 ACH , 1 CH _{3} | |
| CCN | CH _{3} CN | 1.0731 | 0.3539 | 2,5-Methylpyridine: 1 AC _{2} N , 3 ACH , 2 CH _{3} |
| CH _{2} CN | 1.5575 | 1.5193 | Acetonitrile | |
| COOH | COOH | 1.5575 | 1.1666 | Propionitrile: 1 CH _{2} CN , 1 CH _{3} |
| HCOOH | HCOOH | 0.8 | 0.9215 | Acetic acid: 1 COOH , 1 CH _{3} |
| 0.8 | 1.2742 | Formic acid | ||
| CCl | CH _{2} Cl | 0.9919 | 1.3654 | 1-Chlorobutane: 1 CH _{2} Cl , 1 CH _{3}, 2 CH _{2} |
| CHCl | 0.9919 | 1.0127 | 2-Chloropropane: 1 CHCl , 2 CH _{3} | |
| CCl | 0.9919 | 0.66 | tert-Butyl chloride: 1 CHCl , 3 CH _{3} | |
| CCl _{2} | CH _{2} Cl _{2} | 1.8 | 2.5 | Dichloromethane: 1 CH _{2} Cl _{2} |
| CHCl _{2} | 1.8 | 2.1473 | 1,1-Dichloroethane: 1 CHCl _{2}, 1 CH _{3} | |
| CCl _{2} | 1.8 | 1.7946 | 2,2-Dichloropropane: 1 CCl _{2}, 2 CH _{3} | |
| CCl _{3} | CHCl _{3} | 2.45 | 2.8912 | Chloroform |
| CCl _{3} | 2.65 | 2.3778 | 1,1,1-Trichloroethane: 1 CCl _{3}, 1 CH _{3} | |
| CCl _{4} | CCl _{4} | 2.618 | 3.1836 | Tetrachloromethane |
| ACCl | ACCl | 0.5365 | 0.3177 | Chlorobenzene: 1 ACCl, 5 ACH |
| CNO _{2} | CH _{3} NO _{2} | 2.644 | 2.5 | Nitromethane |
| CH _{2} NO _{2} | 2.5 | 2.304 | 1-Nitropropane: 1 CH _{2} NO _{2}, 1 CH _{3}, 1 CH _{2} | |
| CHNO _{2} | 2.887 | 2.241 | 2-Nitropropane: 1 CHNO _{2}, 2 CH _{3} | |
| ACNO _{2} | ACNO _{2} | 0.4656 | 0.3589 | Nitrobenzene: 1 ACNO _{2}, 5 ACH |
| CS _{2} | CS _{2} | 1.24 | 1.068 | Carbon disulfide |
| CH _{3} SH | CH _{3} SH | 1.289 | 1.762 | Methanethiol |
| CH _{2} SH | 1.535 | 1.316 | Ethanethiol: 1 CH _{2} SH , 1 CH _{3} | |
| Furfural | furfural | 1.299 | 1.289 | Furfural |
| Diol | \left( CH _{2} OH \right)_{2} | 2.088 | 2.4 | 1,2-Ethanediol (ethylene glycol) |
| I | I | 1.076 | 0.9169 | Ethyl iodide: 1 I , 1 CH _{3}, 1 CH _{2} |
| Br | Br | 1.209 | 1.4 | Ethyl bromide: 1 Br , 1 CH _{3}, 1 CH _{2} |
| C \equiv C | CH \equiv C | 0.9214 | 1.3 | 1-Hexyne: 1 CH \equiv C , 1 CH _{3}, 3 CH _{2} |
| C \equiv C | 1.303 | 1.132 | 2-Hexyne: 1 C \equiv C , 2 CH _{3}, 2 CH _{2} | |
| DMSO | DMSO | 3.6 | 2.692 | Dimethyl sulfoxide |
| ACRY | ACRY | 1 | 0.92 | Acrylonitrile |
| Cl ( C = C ) | Cl ( C = C ) | 0.5229 | 0.739 | Trichloroethylene: 3 Cl(C=C), 1 CH=C |
| ACF | ACF | 0.8814 | 0.7269 | Hexafluorobenzene: 6 ACF |
| DMF | DMF | 2 | 2.093 | N,N-Dimethylformamide |
| HCON \left( CH _{2}\right)_{2} | 2.381 | 1.522 | N,N-Diethylformamide: 1 HCON \left( CH _{2}\right)_{2}, 2 CH _{3} | |
| CF _{2} | CF _{3} | 1.284 | 1.266 | 1,1,1-Trifluoroethane: 1 CF _{3}, 1 CH _{3} |
| CF _{2} | 1.284 | 1.098 | Perfluorohexane: 4 CF _{2}, 2 CF _{3} | |
| CF | 0.8215 | 0.5135 | Perfluoromethylcyclohexane: 1 CF , 5 CF _{2}, 1 CF _{3} | |
| COO | COO | 1.6 | 0.9 | Methyl acrylate: 1 COO , 1 CH _{3}, 1 CH _{2}= CH |
| cy – CH _{2} | cy – CH _{2} | 0.7136 | 0.8635 | Cyclohexane: 6 cy – CH _{2} |
| cy – CH | 0.3479 | 0.1071 | Methylcyclohexane: 1 cy – CH , 5 cy – CH _{2}, 1 CH _{3} | |
| cy-C | 0.347 | 0 | 1,1-Dimethylcyclohexane: 1 cy – C , 5 cy – CH _{2}, 2 CH _{3} | |
| cy – CH _{2} O | cy – CH _{2} OCH | 1.7023 | 1.8784 | Tetrahydrofuran: 1 cy – CH _{2} OC H _{2}, 2 cy – CH _{2} |
| \text { cy-CH } C _{2} O \left( CH _{2}\right)_{1 / 2} | 1.4046 | 1.4 | 1,3-Dioxane: 2 cy – CH _{2} O \left( CH _{2}\right)_{1 / 2}, 1 \text { cy-CH } CH _{2} | |
| \text { cy- }\left( CH _{2}\right)_{1 / 2} O \left( CH _{2}\right)_{1 / 2} | 1.0413 | 1.0116 | 1,3,5-Trioxane: 3 cy -\left( CH _{2}\right)_{1 / 2} O \left( CH _{2}\right)_{1 / 2} | |
| cy-CON-C | cy – CON – CH _{3} | 3.9819 | 3.2 | N-Methylpyrrolidone: 1 cy – CON – CH _{3}, 3 cy – CH _{2} |
| cy – CON – CH _{2} | 3.7543 | 2.892 | N-Ethylpyrrolidone: 1 \text { cy-CON-CH }{ }_{2}, 3 \text { cy- } CH _{2}, 1 CH _{3} | |
| cy-CON-CH | 3.5268 | 2.58 | N-Isopropylpyrrolidone: 1 \text { cy-CON-CH, } 3 \text { су-CH } H _{2}, 2 CH _{3} | |
| cy-CON-C | 3.2994 | 2.352 | N-tert-Butylpyrrolidone: 1 \text { cy-CON-C, } 3 \text { cy-CH } CH _{2}, 3 CH _{3} | |
| ACS | AC _{2} H _{2} S | 1.7943 | 1.34 | Thiophene: 1 AC _{2} H _{2} S , 2 ACH |
| AC _{2} HS | 1.6282 | 1.06 | 2-Methylthiophene: 1 AC _{2} HS , 2 ACH , 1 CH _{3} | |
| AC _{2} S | 1.4621 | 0.78 | 2,5-Dimethylthiophene: 1 AC _{2} S , 2 ACH , 2 CH _{3} | |
| Note: A (as in ACH) denotes a group in an aromatic ring, cy- denotes a group in a cyclic structure, and functional groups in bold without any example assignments, such as water, formic acid, etc. are specific to that molecule. | ||||