Question 10.11: Nonequilibrium Solidification of Cu-Ni Alloys Calculate the ......
Nonequilibrium Solidification of Cu-Ni Alloys
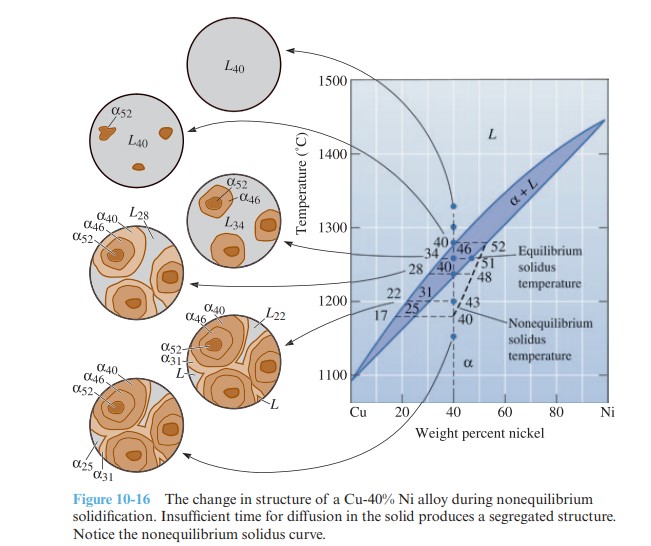
Calculate the composition and amount of each phase in a Cu-40% Ni alloy that is present under the nonequilibrium conditions shown in Figure 10-16 at 1300 °C, 1280 °C, 1260 °C, 1240 °C, 1200 °C, and 1150 °C. Compare with the equilibrium compositions and amounts of each phase.
Learn more on how do we answer questions.
We use the tie line to the equilibrium solidus temperature curve to calculate compositions and percentages of phases as per the lever rule. Similarly, the nonequilibrium solidus temperature curve is used to calculate percentages and concentrations of different phases formed under nonequilibrium conditions.
|
Temperature |
Equilibrium |
Nonequilibrium |
| 1300 °C | L: 40% Ni 100% L | L: 40% Ni 100% L |
| 1280 °C | L: 40% Ni 100% L | L: 40% Ni 100% L |
| 1260 °C |
L: 34% Ni \frac{46 \ – \ 40}{46 \ – \ 34}= 50% L
α: 46% Ni\frac{40 \ – \ 34}{46 \ – \ 34}= 50% α |
L: 34% Ni \frac{51 \ – \ 40}{51 \ – \ 34}= 65% L
α: 51% Ni\frac{40 \ – \ 34}{51 \ – \ 34}= 35% α |
| 1240 °C |
L: 28% Ni ∼ 0% L
α: 40% Ni 100% α |
L: 28% Ni \frac{48 \ – \ 40}{48 \ – \ 28}= 40% L
α: 48% Ni\frac{40 \ – \ 28}{48 \ – \ 28}= 60% α |
| 1200 °C |
α: 40% Ni 100% α |
L: 22% Ni \frac{43 \ – \ 40}{43 \ – \ 22}= 14% L
α: 43% Ni\frac{40 \ – \ 22}{43 \ – \ 22}= 86% α |
| 1150 °C | α: 40% Ni 100% α | α: 40% Ni 100% α |