Question 10.10: Using VSEPR Theory to Predict a Geometric Shape Predict the ...
Using VSEPR Theory to Predict a Geometric Shape
Predict the molecular geometry of the polyatomic anion ICl_4{ }^-.
Analyze
To solve this problem, apply the four steps outlined on page 428.
Learn more on how we answer questions.
Step 1. Write the Lewis structure. The number of valence electrons is
\begin{matrix} \text{From I} & \text{From Cl} & \text{To establish ionic charge of } -1 & \\ (1 \times 7) & (4 \times 7) + & 1 & = 36\end{matrix}
To join 4 Cl atoms to the central I atom and to provide octets for all the atoms, we need 32 electrons. In order to account for all 36 valence electrons, we need to place an additional four electrons around the I atom as lone pairs. That is, we are forced to expand the valence shell of the I atom to accommodate all the electrons required in the Lewis structure.
\left[\begin{array}{r c}\begin{matrix} \ \text{:}\underset{\cdot \cdot}{\ddot{Cl} } \ _{_\diagdown }\quad\quad\quad _{_\diagup }\underset{\cdot \cdot}{\ddot{Cl} }\text{:} \\\text{:I:}\\ \text{:}\underset{\cdot \cdot}{\ddot{Cl} }\ ^{^\diagup }\quad \quad \ \ ^{^\diagdown }\underset{\cdot \cdot}{\ddot{Cl} }\text{:} \\ \\ \end{matrix} \end{array} \right]^-
Step 2. There are six electron groups around the I atom, four bond pairs and two lone pairs.
Step 3. The electron-group geometry (the orientation of six electron groups) is octahedral.
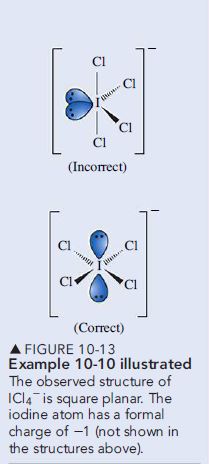
Step 4. The ICl_4{ }^- anion is of the type A {\chi} _4 E_2, which according to Table 10.1, leads to a molecular geometry that is square planar.
| TABLE 10.1 Molecular Geometry as a Function of Electron-Group Geometry | ||||||
| Number of Electron Groups | Electron-Group Geometry | Number of Lone Pairs | VSEPR Notation | Molecular Geometry | Ideal Bond Angles | Example |
| 2 | linear | 0 | A \chi_2 |  |
180° | BeCl_2  |
| 3 | trigonal planar | 0 | A \chi_3 |  |
120° | BF_3  |
| trigonal planar | 1 | A \chi_2 E |  |
120° | SO_2{ }^a | |
| 4 | tetrahedral | 0 | A \chi_4 |  |
109.5° | CH_4  |
| tetrahedral | 1 | A \chi_3 E |  |
109.5° | NH_3 | |
| tetrahedral | 2 | A \chi_2 E_2 |  |
109.5° | OH_2 | |
| 5 | trigonal bipyramidal | 0 | A \chi_5 |  |
90°,120° | PCl_5  |
| trigonal bipyramidal | 1 | A \chi_4 E^b |  |
90°,120° | SF_4 | |
| trigonal bipyramidal | 2 | A \chi_3 E_2 |  |
90° | ClF_3 | |
| trigonal bipyramidal | 3 | A \chi_2 E_3 |  |
180° | \chi eF_2 | |
| 6 | octahedral | 0 | A \chi_6 |  |
90° | SF_6  |
| octahedral | 1 | A \chi_5 E |  |
90° | BrF_5 | |
| octahedral | 2 | A \chi_4 E_2 |  |
90° | \chi eF_4 | |
_{}^{a}\textrm{For }a discussion of the structure of SO_2, see page 428.
_{}^{b}\textrm{For }a discussion of the placement of the lone-pair electrons in this structure, see page 427.
Assess
Figure 10-13 suggests two possibilities for distributing bond pairs and lone pairs in ICl_4{ }^-. The square planar structure is correct because the lone pair lone pair interaction is kept at 180°. In the incorrect structure, this interaction is at 90°. which results in a strong repulsion.