Question 15.15: For each of the following reactions, identify the Lewis acid......
For each of the following reactions, identify the Lewis acid and the Lewis base.
(a) CO_{2} + OH^{-}\longrightarrow HCO_{3} ^{-}
(b) B(OH)_{3} + OH^{-}\longrightarrow B(OH)_{4} ^{-}
(c) 6 CN^{-} + Fe^{3+}\longrightarrow Fe(CN)_{6} ^{3-}
STRATEGY
To identify the Lewis acid and the Lewis base, determine which molecule or ion can accept an electron pair (the Lewis acid) and which can donate an electron pair (the Lewis base).
Learn more on how do we answer questions.
(a) The carbon atom of O=C=O bears a partial positive charge (δ+) because carbon is less electronegative than oxygen. Therefore, the carbon atom attracts an electron pair from OH^{-}. Formation of a covalent bond from OH^{-} to CO_{2} is helped along by a shift of a shared electron pair to oxygen:
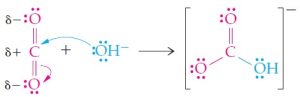
The Lewis acid (electron-pair acceptor) is CO_2; the Lewis base (electron-pair donor) is OH^-.
(b) The Lewis acid is boric acid, B(OH)_{3}, a weak acid and mild antiseptic used in eyewash. The boron atom in B(OH)_{3} has a vacant valence orbital and completes its octet by accepting a pair of electrons from the Lewis base, OH^{-}.
(c) The Lewis acid is Fe^{3+}, and the Lewis base is CN^{-}. Each of the six :C\equiv N:^{-} ions bond to the Fe^{3+} ion by donating a lone pair of electrons on the C atom.