Question 5.SSE.1: (i) Ethanol → (ii) Predict the structure of the product in t......
(i) Ethanol \overset{I _2, NaOH}{\xrightarrow {\hspace{2 cm}} }
(ii) Predict the structure of the product in the following reaction:

\overset{\text {NaI}}{\underset{\text { Acetone}}{\xrightarrow {\hspace{2 cm}} }} \text {C}
[IIT, 1996]
(iii) ( COOH )_2+\left( CH _2 OH \right)_2+\text { conc. } H _2 SO _4 \longrightarrow \text (F)
[IIT, 1997]
(iv) (CH_3)_2CHOCH_3 \overset{\text {HI (excess), heat}} {\xrightarrow {\hspace{2 cm}} } \text {2 Product}
[IIT, 1998]
(v) 1-propanol from 2-propanol (in three steps)
(vi) Ethyl alcohol to vinyl acetate (in mot more than 6 steps)
(vii) Phenol to acetophenone
(viii) Acetic acid to tertiary butyl alcohol.
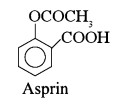
(ix)  \begin{matrix} \longrightarrow & \text {Aspirin} \\\\\\\\ \end{matrix}
\begin{matrix} \longrightarrow & \text {Aspirin} \\\\\\\\ \end{matrix}
[IIT, 2003]
Learn more on how do we answer questions.
CH _3 CHO +3 I _2+4 NaOH \overset{\text {haloform reaction}}{\xrightarrow {\hspace{2 cm}} } HCOONa + CHI _3+3 H _2 O +3 NaI
Br is replaced by I following SN^2 mechanism. There will be Walden inversion at the place of replacement.
 \overset{\text {NaI}}{\underset{\text { Acetone}}{\xrightarrow {\hspace{2 cm}} }}
\overset{\text {NaI}}{\underset{\text { Acetone}}{\xrightarrow {\hspace{2 cm}} }} 
\begin{matrix} O \\ & \diagdown \diagdown \\ && C & – & OH \\ && | \\ & &C & – & OH \\ & \diagup \diagup \\ O\end{matrix} + \begin{matrix} O&HCH_2 \\ |\\ O&HCH_2\end{matrix} \overset{\text {Cone. } H_2SO_4} {\xrightarrow {\hspace{2 cm}} } \\ \quad \quad \quad \quad \quad \quad \quad \quad \quad \begin{matrix} O \\&&&& O \\ & \diagdown \diagdown & & \diagup && \diagdown \\ && C &&&& CH_2 \\ &&|&&&&| \\ &&C &&&& CH_2 \\ & \diagup \diagup &&\diagdown &&\diagup \\ O &&&& O \end{matrix}
\begin{matrix} H_3C &&&&&&&& H_3C \\ & \diagdown &&&&&&&& \diagdown \\ && CH & – & O & – & CH_3 & \overset{\text {HI (excess)}} {\xrightarrow {\hspace{2 cm}} } & && CHOH & + & CH_3I \\ & \diagup &&&&&&&& \diagup \\ H_3C &&&&&&&& H_3C \end{matrix}
\begin{matrix} CH _3 CH ( OH ) CH _3 & \overset{\text { conc. } H _2 SO _4}{\underset{- H _2 O}{\xrightarrow {\hspace{2 cm}} }} & CH _3 CH = CH _2 & \overset{\text { HBr }}{\xrightarrow {\hspace{2 cm}} } & CH _3 CH _2 CH _2 Br & \overset{\text { aq. } NaOH}{\xrightarrow {\hspace{2 cm}} } & CH _3 CH _2 CH _2 OH\\ \text {2-propanol} &&&&&& \text {1-propanol}\end{matrix}
\begin{matrix} CH _3 CH _2 OH & \overset{Al _2 O _3, 350^{\circ} C}{\xrightarrow {\hspace{2 cm}} } & CH _2= CH _2 & \overset{Br_2}{\xrightarrow {\hspace{2 cm}} } & BrCH _2 \cdot CH _2 Br & \overset{\text { alc. } KOH}{\xrightarrow {\hspace{2 cm}} } & CH \equiv CH & \overset{CII _3 COOII}{\xrightarrow {\hspace{2 cm}} } & CH _2= CHOCOCH _3 \\ \text {Ethanol} &&&&&&&& \text {Vinyl acetate} \end{matrix}
\begin{matrix} C _6 H _5 OH & \overset{Zn}{\underset{\text {distillation}}{\xrightarrow {\hspace{2 cm}} }} & C_6H_6 & \overset{CH _3 COCl}{\underset{\text { anhy. } AlCl _3}{\xrightarrow {\hspace{2 cm}} }} & C _6 H _5 COCH _3 \\ \text {Phenol} &&&& \text {Acetophenone} \end{matrix}
\begin{matrix} CH _3 COOH & \overset{PCl_3}{\xrightarrow {\hspace{2 cm}} } & CH _3 COCl & \overset{CH _3 Cl}{\underset{CH _3 MgI}{\xrightarrow {\hspace{2 cm}} }} \\ \text {Acetic acid} \end{matrix} \begin{matrix}&& H_3C &&&& CH_3 && H_3C &&&& CH_3\\ &&& \diagdown && \diagup &&&& \diagdown &&\diagup \\ CH _3 COCH _3 & \longrightarrow & && C &&& \overset{H_2O}{\underset{H^-}{\xrightarrow {\hspace{2 cm}} }} &&& C\\ &&&\diagup &&\diagdown &&&& \diagup && \diagdown \\ && H_3C &&&& OMgI && H_3C &&&& OH \\ &&&&&&&&&& \text {Tertiary butyl alcohol} \end{matrix}
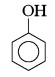 \begin{matrix} \overset{\text { (i) } NaOH}{\underset{-H_2O}{\xrightarrow {\hspace{2 cm}} }} \\\\\\\\ \end{matrix}
\begin{matrix} \overset{\text { (i) } NaOH}{\underset{-H_2O}{\xrightarrow {\hspace{2 cm}} }} \\\\\\\\ \end{matrix}  \begin{matrix} \overset{CO _2, 140^{\circ} C , 6 \text { atm}}{\underset{\text { (ii) } H}{\xrightarrow {\hspace{2 cm}} }} \\\\\\\\ \end{matrix}
\begin{matrix} \overset{CO _2, 140^{\circ} C , 6 \text { atm}}{\underset{\text { (ii) } H}{\xrightarrow {\hspace{2 cm}} }} \\\\\\\\ \end{matrix}
 \begin{matrix} \overset{\left( CH _3 CO \right)_2 O}{\xrightarrow {\hspace{2 cm}} } \\\\\\\\ \end{matrix}
\begin{matrix} \overset{\left( CH _3 CO \right)_2 O}{\xrightarrow {\hspace{2 cm}} } \\\\\\\\ \end{matrix}