Question 14.1: Entropy Maximum Principle A 2 kg copper block at 100°C is br...
Entropy Maximum Principle
A 2 kg copper block at 100°C is brought in thermal contact with a 5 kg aluminum block at 50°C. Treating the combined system as an isolated system, determine the final temperature (a) by assuming that at equilibrium the temperatures of the two blocks are equal and (b) by using the entropy maximum principle, without assuming the final temperatures to be equal.
Learn more on how we answer questions.
Perform an energy and entropy analysis of the closed process executed by the combined non-uniform system within the red boundary of Figure 14.3.
Assumptions
The SL model is applicable to the subsystems and a composite state made of two local states in LTE can describe the global beginning and final states. No changes in KE or PE.
Analysis
The copper block is sub-system A and the aluminum block is sub-system B. From Table A-1, or any SL TESTcalc, we obtain c_{v A}=0.386 kJ / kg \cdot K \text { and } c_{v B}=0.90 kJ / kg \cdot K . Designating the composite beginning state (bA and bB states) by States 1 and 2 and the composite final state ( fA and fB states) by States 3 and 4 (Fig. 14.3), the energy equation for the closed process, Eq. (5.2), can be simplified:
\underbrace{\Delta E=E_{f}-E_{b}}_{\begin{array}{l}\text { Change in } E \text { during } \\\text { the process. }\end{array}}=\underbrace{Q}_{\begin{array}{l}\text { Heat added to } \\\text { the system. }\end{array}}-\underbrace{W_{\text {ext }}}_{\begin{array}{l}\text { Work transferred } \\\text { out of the system. }\end{array}} ; \quad[ kJ ] (5.2)
\begin{aligned}&\Delta U=\cancel{Q}^{0}-\cancel{W}^{0} ; \quad \Rightarrow \quad \Delta\left(U_{A}+U_{B}\right)=0 ; \quad \Rightarrow \quad \Delta U_{A}=-\Delta U_{B} ;\\&\Rightarrow \quad m_{A} c_{v A}\left(T_{f A}-T_{b A}\right)=m_{B} c_{v B}\left(T_{b B}-T_{f B}\right) \text {; }\\&\Rightarrow \quad m_{A} c_{v A}\left(T_{3}-T_{1}\right)=m_{B} c_{v B}\left(T_{2}-T_{4}\right)\end{aligned}
Substituting the known variables, the energy equation reduces to:
T_{3}=2256-5.83 T_{4} (14.3)
Given (and also known from our experience) that T_{4}=T_{3}=T_{f} , Eq. (14.3) produces T_{f} as 330.3 K or 57.3°C.
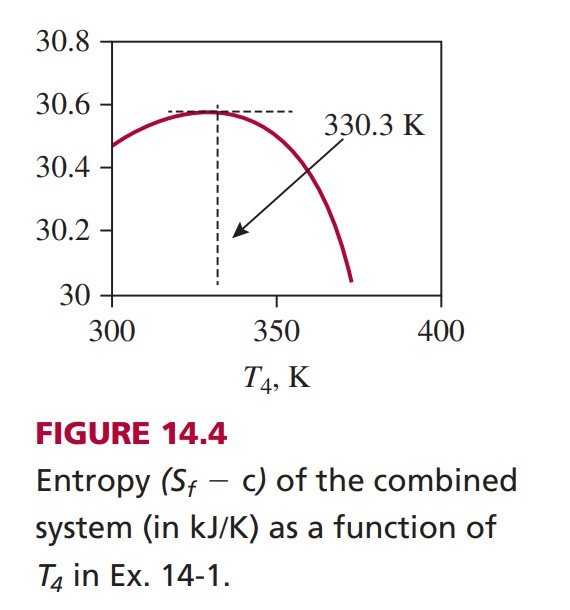
(b) The entropy of the combined system, which is isolated, reaches a maximum at equilibrium. To locate the maximum, one approach is to plot the entropy of the combined system against all possible final states. The entropy of the composite system can be expressed in terms of T_{3} \text { and } T_{4} using the SL model:
\begin{aligned}&S_{f}-S_{b}=\left(S_{f A}+S_{f B}\right)-\left(S_{b A}+S_{b B}\right) \\&\Rightarrow \quad \Delta S=\left(S_{f A}-S_{b A}\right)+\left(S_{f B}-S_{b B}\right)=m_{A} c_{v A} \ln \frac{T_{f A}}{T_{b A}}+m_{B} c_{v B} \ln \frac{T_{f B}}{T_{b B}} \\&\Rightarrow \quad S_{f}=\left(S_{b}-m_{A} c_{v A} \ln T_{1}-m_{B} c_{v B} \ln T_{2}\right)+m_{A} c_{v A} \ln T_{3}+m_{B} c_{v B} \ln T_{4}\end{aligned}
Representing the terms inside the parentheses by a constant c, and substituting the constraint from the energy equation, Eq. (14.3), the final entropy can be expressed as a function of T_{4} alone:
S_{f}= c +0.772 \ln \left(2256-5.83 T_{4}\right)+4.5 \ln T_{4} \quad [kJ/K] (14.4)
( S_{f} – c), evaluated from this expression, is plotted against T_{4} (which represents the possible final temperature of sub-system B) in Figure 14.4. To obtain the precise temperature at which the entropy peaks, set the derivative of S_{f} , with respect to T_{4} , to zero and solve the resulting equation to show that T_{4}=57.3^{\circ} C at the maximum. This result is consistent with the maximum exhibited by the graph in Figure 14.4.
TEST Analysis
Launch the SL/SL non-uniform, non-mixing, closed-process TESTcalc. Evaluate State-1 and State-2 with the given information (mass and temperature) and leave the temperatures unknown for State-3 and State-4. For each state, make sure that the working substance is correctly selected (there are two working substance selectors, the left one can be used for sub-system A and the other one for sub-system B). In the process panel, set up the adiabatic process by selecting the composite anchor states and entering process variables Q=W_ext=0. Now guess a value of T3, say, 75 deg-C, and click Super-Calculate. The entropy change of the system, Delta_S, is calculated as 0.0058 kJ/K. Change T3 to a new value and Super-Calculate to update the value of Delta_S. It can be verified that when T3 is 57.3 deg-C, entropy change reaches a maximum, and T4 becomes equal to T3. See TEST-code (TEST > TEST-codes) for further details.
Discussion
If the two subsystems consist of two gases separated by a sliding piston, instead of two solid blocks, the entropy maximum principle leads to equality of pressure as well (see Anim. 14.A. mechEquilibrium). While the equality of temperature or pressure among subsystems at equilibrium is almost trivial, our experience falls far short in predicting more challenging equilibrium conditions when there is a phase or chemical transformation involved.