Question 10.9: Representing the Lewis Structure of a Resonance Hybrid Write...
Representing the Lewis Structure of a Resonance Hybrid
Write the Lewis structure of the acetate ion, CH_3 COO^-.
Analyze
A key concept is that resonance structures differ only in how electrons are distributed within the structure. We cannot change the positions of the atoms. First, we draw a skeletal structure (see the electrostatic potential map shown in the margin), and then we complete it by using the strategy we’ve used previously. Finally, we generate additional structures (resonance structures) by moving electron pairs.
Learn more on how we answer questions.
The skeletal structure has the three H atoms as terminal atoms bonded to a C atom as a central atom. The second C atom is also a central atom bonded to the first. The two O atoms are terminal atoms bonded to the second C atom.
\begin{array}{r c} \begin{matrix} \ \ \ \ \ \ \ \ \ \ \ \underset{|}{H} \ \ \ \ \ \underset{|}{O} \ \ \ \ \ \ \ \ \ \ \\ H-C-C-O \\ \ \overset{|}{H} \ \ \ \ \ \ \ \ \ \\ \end{matrix} \end{array}
The number of valence electrons (dots) that must appear in the Lewis structure is
\begin{array}{r c} \begin{matrix} (3 \times 1) & + & (2 \times 4) & + & (2 \times 6) & + & 1 = 3 + 8 + 12 + 1 = 24 \\ \text{From H} & & \text{From C} & & \text{From O} & & \overset{\uparrow}{\text{To}} \text{establish charge of 1-} \end{matrix} \end{array}
Twelve of the valence electrons are used in the bonds in the skeletal structure, and the remaining twelve are distributed as lone-pair electrons on the two O atoms.

In completing the octet of the C atom on the right, we discover that we can write two completely equivalent Lewis structures, depending on which of the two O atoms furnishes the lone pair of electrons to form a carbon-to-oxygen double bond. The true Lewis structure is a resonance hybrid of the following two contributing structures.
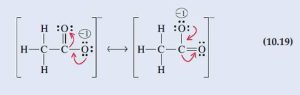
Assess
Even though the formal process of converting one resonance structure to another moves electrons, resonance is not meant to indicate the motion of electrons. The acetate anion has a structure that is a composite of the two resonance forms that we have constructed.
