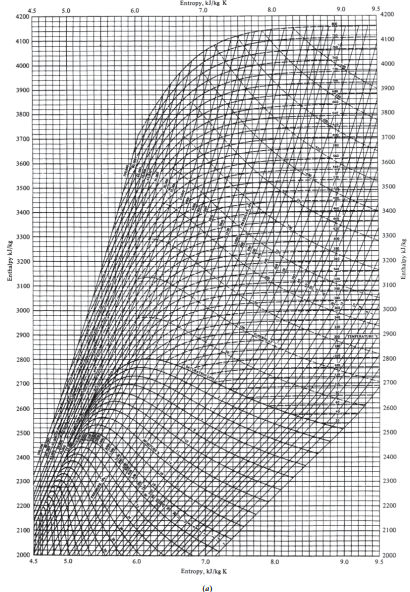
Another Example of Using the Entropy Balance in Problem Solving
A steam turbine operates at the following conditions:
\begin{array}{lccc}\hline & \text { Inlet } & & \text { Outlet } \\\hline \text { Velocity }( m / min ) & 2000 & & 7500 \\T( K ) & 800 & & 440 \\P( MPa ) & 3.5 & & 0.15 \\\text { Flow rate }( kg / hr ) & & 10000 & \\\text { Heat loss }( kJ / hr ) & & 125000 & \\\hline\end{array}a. Compute the horsepower developed by the turbine and the entropy change of the steam.
b. Suppose the turbine is replaced with one that is well insulated, so that the heat loss is eliminated, and well designed, so that the expansion is reversible. If the exit pressure and velocity are maintained at the previous values, what are the outlet steam temperature and the horsepower developed by the turbine?