Question 8.5.2: Identify molecular geometry. Identify molecular geometry Det......
Identify molecular geometry.
Identify molecular geometry
Determine the shape of
a. ICl_{5}
b. SO_{3}^{2-}
Learn more on how do we answer questions.
You are asked to determine the shape of a molecule or ion.
You are given the chemical formula for the molecule or ion.
Draw the Lewis structure of each species, sum the number of structural electron pairs around the central atom, and use Interactive Table 8.5.1 to determine the electron-pair geometry. Use the number of bonding and nonbonding structural pairs, along with Interactive Table 8.5.2, to determine the molecular geometry.
a. ICl_{5} has six structural electron pairs around the central atom. Five are bonding electrons, and one is nonbonding.
\begin{matrix} :\overset{..}{\underset{|}{C}:} \\ \,:\overset{..}{\underset{..}{C}_{\diagdown }} \,\,\,\,\,\,\,\,_{\diagup }\overset{..}{\underset{..}{C}:} \\\underset{..}{I} \\ :\overset{..}{\underset{..}{C}^{\diagup}} \,\,\,\,\,\,\,\,^{\diagdown }\overset{..}{\underset{..}{C}:} \end{matrix} 
The electron-pair geometry is octahedral, and the molecular geometry is square pyramidal.
b. SO_{3}^{2-} has four structural electron pairs around the central atom. Three are bonding electrons, and one is nonbonding.
\left[\begin{matrix} :\overset{..}{\underset{|}{O}:}\\ :\overset{..}{\underset{..}{O}}- {\underset{..}{S}} – \overset{..}{\underset{..}{O}:} \end{matrix} \right]^{2-} 
The electron-pair geometry is tetrahedral, and the molecular geometry is trigonal pyramidal.
Interactive Table 8.5.1
Ideal Electron-Pair Geometries
| Number of Structural Pairs | Species Type (A = central atom, X = terminal atom) | Electron-Pair Geometry | Example | X—A—X Bond Angles |
| 2 | AX_{2} | Linear | CO_{2} | |
| 3 | AX_{3} | Trigonal planar | BF_{3} |  |
| 4 | AX_{4} | Tetrahedral | CH_{4} |  |
| 5 | AX_{5} | Trigonal bipyramidal | PCl_{5} |  |
| 6 | AX_{6} | Octahedral | SF_{6} |  |
Interactive Table 8.5.2
Shapes of Molecules with Three to Six Structural Electron Pairs
| Number of Structural Electron Pairs | Number of Nonbonding Structural Pairs | |||
| 0 | 1 | 2 | 3 | |
| 3 Trigonal planar BF_{3}  |
Bent O_{3}  |
|||
| 4 Tetrahedral CF_{4}  |
Trigonal pyramidal NH_{3}  |
Bent H_{2}O 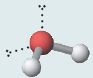 |
||
| 5 Trigonal bipyramidal PF_{5}  |
Seesaw SF_{4}  |
T-shaped ClF_{3}  |
Linear XeF_{2}  |
|
| 6 Octahedral SF_{6} 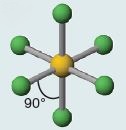 |
Square pyramidal BrF_{5}  |
Square planar XeF_{4}  |
||