Question 24.2: Predicting Nuclear Stability Problem Which of the following ......
Predicting Nuclear Stability
Problem Which of the following nuclides would you predict to be stable and which radioactive: (a) _{10}^{18}Ne; (b) _{16}^{32}S; (c) _{90}^{236}Th; (d) _{56}^{123}Ba? Explain.
Learn more on how do we answer questions.
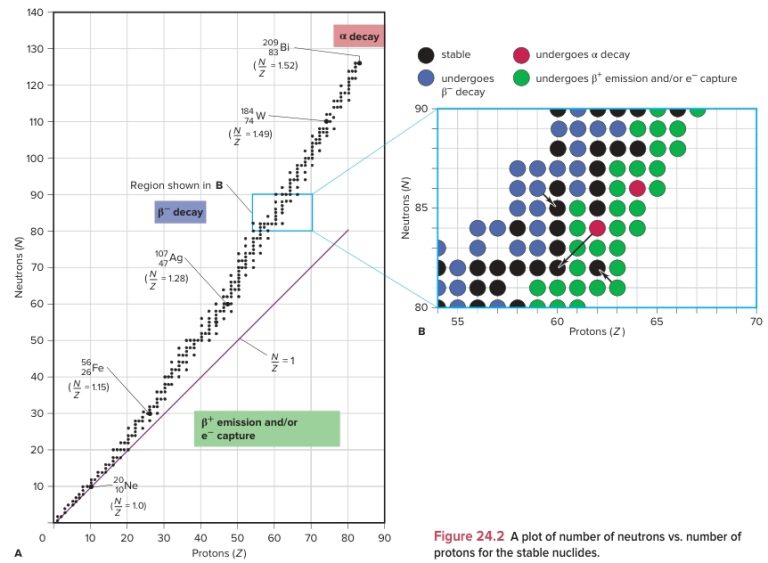
Plan In order to evaluate the stability of each nuclide, we determine the N and Z values, the N/Z ratio from (A − Z)/Z, and the value of Z; then we compare the N/Z ratio with the stable values (from Figure 24.2A) and note whether Z and N are even or odd.
Solution (a) Radioactive. This nuclide has N = 8 (18 − 10) and Z = 10, so N/Z = \frac{18 − 10}{10} = 0.8. Except for hydrogen-1 and helium-3, no nuclides with N < Z are stable; despite its even N and Z, this nuclide has too few neutrons to be stable.
(b) Stable. This nuclide has N = Z = 16, so N/Z = 1.0. With Z < 20 and even N and Z, this nuclide is most likely stable.
(c) Radioactive. This nuclide has Z = 90, and every nuclide with Z > 83 is radioactive.
(d) Radioactive. This nuclide has N = 67 and Z = 56, so N/Z = 1.20. For Z values of 55 to 60, Figure 24.2A shows N/Z ≥ 1.3 for stable nuclides, so this nuclide has too few neutrons to be stable.
Check By consulting a table of isotopes, such as the one in the CRC Handbook of Chemistry and Physics, we find that our predictions are correct.