Question 27.5: Predicting SN2, E2, SN1, and E1 Reactions For each of the fo...
Predicting S_N2, E2, S_N1, and E1 Reactions
For each of the following reactions, predict the products and the mechanisms by which the products are formed.
(a) CH_3CH_2CH_2CH_2Br + (CH_3)_3CO^-Na^+ \xrightarrow{(CH_3)_3COH}
(b) \begin{matrix} \ \underset{|}{Br} \quad\quad\quad\quad\quad\quad \underset{||}O \quad\quad\quad\quad\quad\quad \\ CH_3CHCH_3 + CH_3C-O^-Na^+ \xrightarrow{\text{acetone}} \end{matrix} (c) \begin{matrix} \underset{|}{Br}\quad\quad\quad \\ CH_3CH_2CHCH_2CH_3 \xrightarrow[{80}^{\circ}C]{H_2O} \end{matrix}
Analyze
We follow the six-step approach outlined above and use the decision tree in Figure 27-13 to guide our thinking.

Learn more on how we answer questions.
(a) We identify the electrophile as CH_3CH_3CH_2Br, which is a primary haloalkane. The nucleophile is (CH_3)_3CO^-, a sterically hindered strong base.

Although a strong base is usually a strong nucleophile, the bulkiness of this base disfavors substitution, and we expect that elimination by E2 will provide the major product.
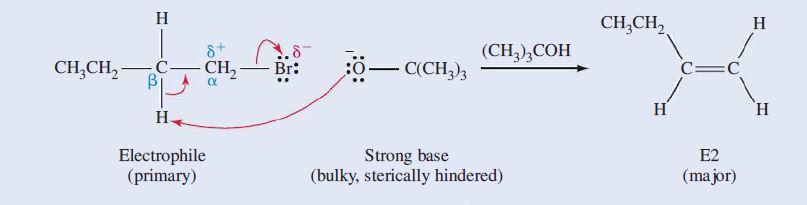
Substitution by S_N2 will yield CH_3CH_2CH_3CH_2OC(CH_3)_3, but the substitution product will be the minor product.
(b) The electrophile is a secondary haloalkane. The electron pair donor is a weak base (the conjugate base of a weak acid) and, because it is negatively charged, it is a reasonably good nucleophile, especially in a polar aprotic solvent. We expect that the main reaction will be S_N2.

(c) Molecules of the solvent also serve as the electron pair donor. The reaction involves a secondary haloalkane with a weak base/weak nucleophile in a polar protic solvent. These conditions do not favor S_N2 or E2 but rather S_N1 and E1. S_N1 and E1 always occur together. Secondary carbocations are relatively stable, especially in a polar protic solvent, such as water. We expect both S_N1 and E1 products. Because H_2O is a very weak base and a fair nucleophile, substitution will dominate over elimination.
\begin{matrix} \underset{|}{Br} \ \quad\quad\quad\quad\quad\quad\quad\quad\quad\quad\quad\quad\quad\quad \underset{|}{OH} \quad\quad\quad\quad\quad\quad\quad\quad\quad\quad\quad\quad\quad\quad\quad\quad\quad \\ CH_3CH_2CHCH_2CH_3 + H_2O \xrightarrow{H_2O} CH_3CH_2CHCH_2CH_3 \text{ or } CH_3CH=CHCH_2CH_3 + HBr \\ \\ \end{matrix}\quad\text{Electrophile} \quad\quad\quad \text{Weak base} \quad\quad\quad\quad\quad\quad S_N1 \quad\quad\quad\quad\quad\quad\quad\quad\quad\quad E1 \quad \\ \quad \ \text{(secondary)} \quad\quad \text{(weak nucleophile)} \quad\quad\quad \text{(major)} \quad\quad\quad\quad\quad\quad\quad\quad \text{(minor)}
Assess
The mechanism for the reaction in (c) was not explicitly shown. We must make sure we are able to show the steps involved in forming the S_N1 and E1 products, including using arrows to show the movement of electrons. Also in (c), (E) and (Z) isomers of 2-pentene are possible.
\begin{matrix} \ H\ _{_\diagdown }\quad\quad\quad _{_\diagup }H \\C=C\\ \quad\quad H_3C\ ^{^\diagup }\quad \quad \ \ ^{^\diagdown }CH_2CH_3 \\ \\ \text{(Z)-2-pentene}\end{matrix} \begin{matrix} H_3C\ _{_\diagdown }\quad\quad\quad _{_\diagup }H \quad \\C=C\\ \quad\quad\quad H\ ^{^\diagup }\quad \quad \ \ ^{^\diagdown }CH_2CH_3 \\ \\ \text{(E)-2-pentene}\end{matrix}