Question 8.5.1: Identify electron-pair geometry. Determine the electron-pai......
Identify electron-pair geometry.
Determine the electron-pair geometry of
a. the carbon atoms in acetic acid, CH_{3}CO_{2}H
b. SF_{4}
Learn more on how do we answer questions.
You are asked to determine the electron-pair geometry for a molecule or for specific atoms in a complex molecule.
You are given the chemical formula for the molecule.
Draw the Lewis structure of each species, sum the number of structural electron pairs around the central atom, and use Interactive Table 8.5.1 to determine the electron-pair geometry.
a. The Lewis structure of acetic acid shows that there are four structural electron pairs (four single bonds) around the carbon on the left and three structural electron pairs (two single bonds and one double bond) around the carbon on the right.
The electron-pair geometry is tetrahedral around the carbon on the left (bond angles of 109.5º) and trigonal planar around the carbon on the right (bond angles of 120º).
b. There are five structural electron pairs around the central atom in S_{4}: four single bonds and one lone pair of electrons.
The electron-pair geometry of SF_{4} is trigonal bipyramidal.
Table 8.5.1
Ideal Electron-Pair Geometries
| Number of Structural Pairs |
Species Type (A = central atom, X = terminal atom) | Example | Example | X-A-X Bond Angles |
| 2 | AX_{2} | Linear | CO_{2} |  |
| 3 | AX_{3} | Trigonal planar | BF_{3} | 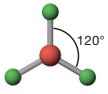 |
| 4 | AX_{4} | Tetrahedral | CH_{4} |  |
| 5 | AX_{5} | Trigonal bipyramidal | PCl_{5} |  |
| 6 | AX_{6} | Octahedral | SF_{6} |  |